An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3
- PMID: 11238595
- PMCID: PMC2193395
- DOI: 10.1084/jem.193.5.643
An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3
Abstract
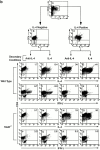
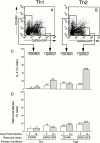
Although interleukin (IL)-12 and IL-4 polarize naive CD4(+) T cells toward T helper cell type 1 (Th1) or Th2 phenotypes, it is not known whether cytokines instruct the developmental fate in uncommitted progenitors or select for outgrowth of cells that have stochastically committed to a particular fate. To distinguish these instructive and selective models, we used surface affinity matrix technology to isolate committed progenitors based on cytokine secretion phenotype and developed retroviral-based tagging approaches to directly monitor individual progenitor fate decisions at the clonal and population levels. We observe IL-4-dependent redirection of phenotype in cells that have already committed to a non-IL-4-producing fate, inconsistent with predictions of the selective model. Further, retroviral tagging of naive progenitors with the Th2-specific transcription factor GATA-3 provided direct evidence for instructive differentiation, and no evidence for the selective outgrowth of cells committed to either the Th1 or Th2 fate. These data would seem to exclude selection as an exclusive mechanism in Th1/Th2 differentiation, and support an instructive model of cytokine-driven transcriptional programming of cell fate decisions.
Figures




Similar articles
-
Altered Th1 cell differentiation programming by CIITA deficiency.J Immunol. 2004 Nov 1;173(9):5501-8. doi: 10.4049/jimmunol.173.9.5501. J Immunol. 2004. PMID: 15494498
-
Enforced expression of GATA-3 in transgenic mice inhibits Th1 differentiation and induces the formation of a T1/ST2-expressing Th2-committed T cell compartment in vivo.J Immunol. 2001 Jul 15;167(2):724-32. doi: 10.4049/jimmunol.167.2.724. J Immunol. 2001. PMID: 11441076
-
Structure and specificity of GATA proteins in Th2 development.Mol Cell Biol. 2001 Apr;21(8):2716-25. doi: 10.1128/MCB.21.8.2716-2725.2001. Mol Cell Biol. 2001. PMID: 11283251 Free PMC article.
-
The function role of GATA-3 in Th1 and Th2 differentiation.Immunol Res. 2003;28(1):25-37. doi: 10.1385/IR:28:1:25. Immunol Res. 2003. PMID: 12947222 Review.
-
'All things considered': transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation.Immunology. 2013 Sep;140(1):31-8. doi: 10.1111/imm.12121. Immunology. 2013. PMID: 23668241 Free PMC article. Review.
Cited by
-
Thrombocytopenia in solid tumors: Prognostic significance.Oncol Rev. 2019 May 14;13(1):413. doi: 10.4081/oncol.2019.413. eCollection 2019 Jan 14. Oncol Rev. 2019. PMID: 31205603 Free PMC article.
-
PPAR and immune system--what do we know?Int Immunopharmacol. 2002 Jul;2(8):1029-44. doi: 10.1016/s1567-5769(02)00057-7. Int Immunopharmacol. 2002. PMID: 12349941 Free PMC article. Review.
-
Development of colitis in signal transducers and activators of transcription 6-deficient T-cell receptor alpha-deficient mice: a potential role of signal transducers and activators of transcription 6-independent interleukin-4 signaling for the generation of Th2-biased pathological CD4+ betabetaT cells.Am J Pathol. 2003 Jan;162(1):263-71. doi: 10.1016/s0002-9440(10)63817-5. Am J Pathol. 2003. PMID: 12507909 Free PMC article.
-
From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation.Immunol Rev. 2010 Nov;238(1):110-25. doi: 10.1111/j.1600-065X.2010.00954.x. Immunol Rev. 2010. PMID: 20969588 Free PMC article. Review.
-
The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo.Clin Exp Immunol. 2004 Nov;138(2):213-20. doi: 10.1111/j.1365-2249.2004.02618.x. Clin Exp Immunol. 2004. PMID: 15498029 Free PMC article.
References
-
- Coffman R.L., Reiner S.L. Instruction, selection, or tampering with the odds? Science. 1999;284:1283. - PubMed
-
- Agarwal S., Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. - PubMed
-
- Murphy K.M., Ouyang W., Farrar J.D., Yang J., Ranganath S., Asnagli H., Afkarian M., Murphy T.L. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000;18:451–494. - PubMed
-
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. - PubMed
-
- Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., Ihle J.N. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

