Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis
- PMID: 11238834
- PMCID: PMC114101
- DOI: 10.1128/JVI.75.7.3077-3088.2001
Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis
Abstract
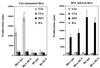
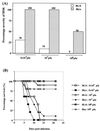
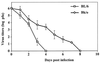
Viruses are suspected but usually unproven triggering factors in autoimmunity. One favored mechanism to explain the role of viruses in the genesis of autoimmunity is molecular mimicry. An immunoinflammatory blinding lesion called herpetic stromal keratitis (HSK) that follows ocular infection with herpes simplex virus (HSV) is suggested to result from a CD4(+) T-cell response to a UL6 peptide of HSV that cross-reacts with a corneal autopeptide shared with the immunoglobulin G2a(b) (IgG2a(b)) isotype. The present report reevaluates the molecular mimicry hypothesis to explain HSK pathogenesis. Our results failed to reveal cross-reactivity between the UL6 and IgG2a(b) peptides or between peptide reactive T cells and HSV antigens. More importantly, animals infected with HSV failed to develop responses that reacted with either peptide, and infection with a recombinant vaccinia UL6 vector failed to cause HSK, in spite of generating UL6 reactivity. Other lines of evidence also failed to support the molecular mimicry hypothesis, such as the failure to affect HSK severity upon tolerization of susceptible BALB/c and B-cell-deficient mice with IgG2a(b) or UL6 peptides. An additional study system revealed that HSK could be induced in mouse strains, such as the OT2 x RAG1(-/-) mice (T cell receptor transgenic recognizing OVA(323-339)) that were unable to produce CD4(+) T-cell responses to any detectable HSV antigens. Our results cast doubt on the molecular mimicry hypothesis as an explanation for the pathogenesis of HSK and indicate that if autoimmunity is involved its likely proceeds via a bystander activation mechanism.
Figures







References
-
- Avery A C, Zhao Z S, Rodriguez A, Bikoff E K, Soheilian M, Foster C S, Cantor H. Resistance to herpes stromal keratitis conferred by an IgG2a derived peptide. Nature. 1995;276:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

