Construction and molecular analysis of gene transfer systems derived from bovine immunodeficiency virus
- PMID: 11238863
- PMCID: PMC114130
- DOI: 10.1128/JVI.75.7.3371-3382.2001
Construction and molecular analysis of gene transfer systems derived from bovine immunodeficiency virus
Abstract
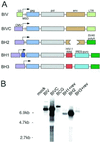
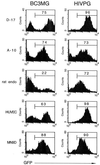
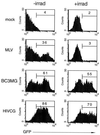
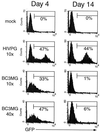
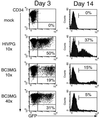
Because lentiviruses are able to infect nondividing cells, these viruses might be utilized in gene therapy applications where the target cell does not divide. However, it has been suggested that the introduction of primate lentivirus sequences, particularly those of human immunodeficiency virus, into human cells may pose a health risk for the patient. To avoid this concern, we have constructed gene transfer systems based on a nonprimate lentivirus, bovine immunodeficiency virus. A panel of vectors and packaging constructs was generated and analyzed in a transient expression system for virion production and maturation, vector expression and encapsidation, and envelope protein pseudotyping. Virion preparations were also analyzed for transduction efficiency in a panel of human and nonhuman primary cells and immortalized cell lines. The virion preparations transduced most of the target cell types, with efficiencies up to 90% and with titers of unconcentrated virus up to 5 x 10(5) infectious doses/ml. In addition, infection of nondividing human cells, including unstimulated hematopoietic stem cells and irradiated endothelial cells, was observed.
Figures


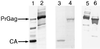

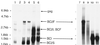
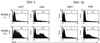
Similar articles
-
Development of second- and third-generation bovine immunodeficiency virus-based gene transfer systems.Hum Gene Ther. 2002 Jul 20;13(11):1293-303. doi: 10.1089/104303402760128522. Hum Gene Ther. 2002. PMID: 12162812
-
Efficient transduction of nondividing cells by optimized feline immunodeficiency virus vectors.Mol Ther. 2000 Jan;1(1):31-8. doi: 10.1006/mthe.1999.0007. Mol Ther. 2000. PMID: 10933909
-
Generation of a packaging cell line for prolonged large-scale production of high-titer HIV-1-based lentiviral vector.J Gene Med. 2005 Jun;7(6):818-34. doi: 10.1002/jgm.726. J Gene Med. 2005. PMID: 15693055
-
Gene therapy: promises and problems.Annu Rev Genomics Hum Genet. 2001;2:177-211. doi: 10.1146/annurev.genom.2.1.177. Annu Rev Genomics Hum Genet. 2001. PMID: 11701648 Review.
-
Vectors derived from the human immunodeficiency virus, HIV-1.Front Biosci. 2003 May 1;8:d491-510. doi: 10.2741/939. Front Biosci. 2003. PMID: 12700118 Review.
Cited by
-
Gene transfer to the outflow tract.Exp Eye Res. 2017 May;158:73-84. doi: 10.1016/j.exer.2016.04.023. Epub 2016 Apr 27. Exp Eye Res. 2017. PMID: 27131906 Free PMC article. Review.
-
In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins.J Virol. 2002 Sep;76(18):9378-88. doi: 10.1128/jvi.76.18.9378-9388.2002. J Virol. 2002. PMID: 12186920 Free PMC article.
-
Comparison of gene transfer efficiencies and gene expression levels achieved with equine infectious anemia virus- and human immunodeficiency virus type 1-derived lentivirus vectors.J Virol. 2002 Feb;76(3):1510-5. doi: 10.1128/jvi.76.3.1510-1515.2002. J Virol. 2002. PMID: 11773424 Free PMC article.
-
Lentiviral Vectors and Adeno-Associated Virus Vectors: Useful Tools for Gene Transfer in Pain Research.Anat Rec (Hoboken). 2018 May;301(5):825-836. doi: 10.1002/ar.23723. Epub 2018 Jan 24. Anat Rec (Hoboken). 2018. PMID: 29149775 Free PMC article. Review.
-
Gene Therapy in Diabetic Retinopathy and Diabetic Macular Edema: An Update.J Clin Med. 2025 May 6;14(9):3205. doi: 10.3390/jcm14093205. J Clin Med. 2025. PMID: 40364236 Free PMC article. Review.
References
-
- Adra C N, Boer P H, McBurney M W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. - PubMed
-
- Berkowitz R D, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. - PubMed
-
- Berkowitz R D, Ilves H, Plavec I, Veres G. Gene transfer systems derived from Visna virus: analysis of virus production and infectivity. Virology. 2001;279:116–129. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

