Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53
- PMID: 11238892
- PMCID: PMC86701
- DOI: 10.1128/MCB.21.5.1552-1564.2001
Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53
Abstract
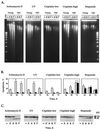
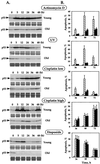
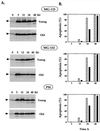
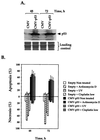
The cellular function of p53 is complex. It is well known that p53 plays a key role in cellular response to DNA damage. Moreover, p53 was implicated in cellular senescence, and it was demonstrated that p53 undergoes modification in senescent cells. However, it is not known how these modifications affect the ability of senescent cells to respond to DNA damage. To address this question, we studied the responses of cultured young and old normal diploid human fibroblasts to a variety of genotoxic stresses. Young fibroblasts were able to undergo p53-dependent and p53-independent apoptosis. In contrast, senescent fibroblasts were unable to undergo p53-dependent apoptosis, whereas p53-independent apoptosis was only slightly reduced. Interestingly, instead of undergoing p53-dependent apoptosis, senescent fibroblasts underwent necrosis. Furthermore, we found that old cells were unable to stabilize p53 in response to DNA damage. Exogenous expression or stabilization of p53 with proteasome inhibitors in old fibroblasts restored their ability to undergo apoptosis. Our results suggest that stabilization of p53 in response to DNA damage is impaired in old fibroblasts, resulting in induction of necrosis. The role of this phenomenon in normal aging and anticancer therapy is discussed.
Figures




Similar articles
-
Inhibition of RNA polymerase II as a trigger for the p53 response.Oncogene. 1999 Jan 21;18(3):583-92. doi: 10.1038/sj.onc.1202356. Oncogene. 1999. PMID: 9989808
-
Evidence that the death receptor DR4 is a DNA damage-inducible, p53-regulated gene.J Cell Physiol. 2001 Jul;188(1):98-105. doi: 10.1002/jcp.1101. J Cell Physiol. 2001. PMID: 11382926
-
ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase.EMBO J. 1997 Oct 1;16(19):6018-33. doi: 10.1093/emboj/16.19.6018. EMBO J. 1997. PMID: 9312059 Free PMC article.
-
[Relationships between p53 induction, cell cycle arrest and survival of normal human fibroblasts following DNA damage].Bull Cancer. 1997 Nov;84(11):1007-16. Bull Cancer. 1997. PMID: 9536982 Review. French.
-
Cellular senescence in cancer treatment: friend or foe?J Clin Invest. 2004 Jan;113(2):169-74. doi: 10.1172/JCI20784. J Clin Invest. 2004. PMID: 14722606 Free PMC article. Review.
Cited by
-
Androgen deprivation-induced senescence promotes outgrowth of androgen-refractory prostate cancer cells.PLoS One. 2013 Jun 28;8(6):e68003. doi: 10.1371/journal.pone.0068003. Print 2013. PLoS One. 2013. PMID: 23840802 Free PMC article.
-
Effects of high glucose and severe hypoxia on the biological behavior of mesenchymal stem cells at various passages.World J Stem Cells. 2024 Apr 26;16(4):434-443. doi: 10.4252/wjsc.v16.i4.434. World J Stem Cells. 2024. PMID: 38690519 Free PMC article.
-
The sculpting of somatic mutational landscapes by evolutionary forces and their impacts on aging-related disease.Mol Oncol. 2022 Sep;16(18):3238-3258. doi: 10.1002/1878-0261.13275. Epub 2022 Jun 29. Mol Oncol. 2022. PMID: 35726685 Free PMC article. Review.
-
Impacts of Senolytic Phytochemicals on Gut Microbiota: A Comprehensive Review.J Microbiol Biotechnol. 2024 Nov 28;34(11):2166-2172. doi: 10.4014/jmb.2408.08032. Epub 2024 Nov 18. J Microbiol Biotechnol. 2024. PMID: 39603836 Free PMC article. Review.
-
The ATM and ATR inhibitors CGK733 and caffeine suppress cyclin D1 levels and inhibit cell proliferation.Radiat Oncol. 2009 Nov 10;4:51. doi: 10.1186/1748-717X-4-51. Radiat Oncol. 2009. PMID: 19903334 Free PMC article.
References
-
- Afshari C A, Vojta P J, Annab L A, Futreal P A, Willard T B, Barrett J C. Investigation of the role of G1/S cell cycle mediators in cellular senescence. Exp Cell Res. 1993;209:231–237. - PubMed
-
- Aladjem M I, Spike B T, Rodewald L W, Hope T J, Klemm M, Jaenisch R, Wahl G M. ES cells do not activate p53-dependent stress and undergo p53-independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–155. - PubMed
-
- Baker S J, Markowitz S, Fearon E R, Willson J K V, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
