Proteasome inhibition induces nuclear translocation and transcriptional activation of the dioxin receptor in mouse embryo primary fibroblasts in the absence of xenobiotics
- PMID: 11238907
- PMCID: PMC86716
- DOI: 10.1128/MCB.21.5.1700-1709.2001
Proteasome inhibition induces nuclear translocation and transcriptional activation of the dioxin receptor in mouse embryo primary fibroblasts in the absence of xenobiotics
Abstract
The aryl hydrocarbon receptor (AHR) is a transcription factor that is highly conserved during evolution and shares important structural features with the Drosophila developmental regulators Sim and Per. Although much is known about the mechanism of AHR activation by xenobiotics, little information is available regarding its activation by endogenous stimuli in the absence of exogenous ligand. In this study, using embryonic primary fibroblasts, we have analyzed the role of proteasome inhibition on AHR transcriptional activation in the absence of xenobiotics. Proteasome inhibition markedly reduced cytosolic AHR without affecting its total cellular content. Cytosolic AHR depletion was the result of receptor translocation into the nuclear compartment, as shown by transient transfection of a green fluorescent protein-tagged AHR and by immunoblot analysis of nuclear extracts. Gel retardation experiments showed that proteasome inhibition induced transcriptionally active AHR-ARNT heterodimers able to bind to a consensus xenobiotic-responsive element. Furthermore, nuclear AHR was transcriptionally active in vivo, as shown by the induction of the endogenous target gene CYP1A2. Synchronized to AHR activation, proteasome inhibition also induced a transient increase in AHR nuclear translocator (ARNT) at the protein and mRNA levels. Since nuclear levels of AHR and ARNT are relevant for AHR transcriptional activation, our data suggest that proteasome inhibition, through a transient increase in ARNT expression, could promote AHR stabilization and accumulation into the nuclear compartment. An elevated content of nuclear AHR could favor AHR-ARNT heterodimers able to bind to xenobiotic-responsive elements and to induce gene transcription in the absence of xenobiotics. Thus, depending on the cellular context, physiologically regulated proteasome activity could participate in the control of endogenous AHR functions.
Figures
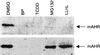




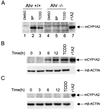
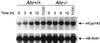


Similar articles
-
Proteasome inhibition induces nuclear translocation of the dioxin receptor through an Sp1 and protein kinase C-dependent pathway.J Mol Biol. 2003 Oct 17;333(2):249-60. doi: 10.1016/j.jmb.2003.08.020. J Mol Biol. 2003. PMID: 14529614
-
Trans-activation by the human aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator proteins: direct interactions with basal transcription factors.Mol Pharmacol. 1996 Sep;50(3):538-48. Mol Pharmacol. 1996. PMID: 8794892
-
Aryl hydrocarbon receptor and aryl hydrocarbon nuclear translocator expression in human and rat placentas and transcription activity in human trophoblast cultures.Toxicol Sci. 2011 Sep;123(1):26-36. doi: 10.1093/toxsci/kfr150. Epub 2011 Jun 10. Toxicol Sci. 2011. PMID: 21666223
-
The aryl hydrocarbon receptor: regulation of hematopoiesis and involvement in the progression of blood diseases.Blood Cells Mol Dis. 2010 Apr 15;44(4):199-206. doi: 10.1016/j.bcmd.2010.01.005. Epub 2010 Feb 19. Blood Cells Mol Dis. 2010. PMID: 20171126 Free PMC article. Review.
-
The search for endogenous activators of the aryl hydrocarbon receptor.Chem Res Toxicol. 2008 Jan;21(1):102-16. doi: 10.1021/tx7001965. Epub 2007 Dec 13. Chem Res Toxicol. 2008. PMID: 18076143 Free PMC article. Review.
Cited by
-
Regulation and function of nuclear IκBα in inflammation and cancer.Am J Clin Exp Immunol. 2012 May 25;1(1):56-66. Print 2012. Am J Clin Exp Immunol. 2012. PMID: 23885315 Free PMC article.
-
Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways.Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9218-23. doi: 10.1073/pnas.0503488102. Epub 2005 Jun 21. Proc Natl Acad Sci U S A. 2005. PMID: 15972329 Free PMC article.
-
Genome-wide B1 retrotransposon binds the transcription factors dioxin receptor and Slug and regulates gene expression in vivo.Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1632-7. doi: 10.1073/pnas.0708366105. Epub 2008 Jan 25. Proc Natl Acad Sci U S A. 2008. PMID: 18223155 Free PMC article.
-
Aryl hydrocarbon receptor blocks aging-induced senescence in the liver and fibroblast cells.Aging (Albany NY). 2022 May 26;14(10):4281-4304. doi: 10.18632/aging.204103. Epub 2022 May 26. Aging (Albany NY). 2022. PMID: 35619220 Free PMC article.
-
New Trends in Aryl Hydrocarbon Receptor Biology.Front Cell Dev Biol. 2016 May 11;4:45. doi: 10.3389/fcell.2016.00045. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27243009 Free PMC article. Review.
References
-
- Adams N H, Levi P E, Hodgson E. Regulation of cytochrome P450 isoenzymes by methylenedioxy phenyl compounds. An updated review of the literature. Rev Biochem Toxicol. 1995;11:205–222.
-
- Alexander D L, Eltom S E, Jefcoate C R. Ah receptor regulation of CYP1B1 expression in primary mouse embryo-derived cells. Cancer Res. 1997;57:4498–4506. - PubMed
-
- Alexander D L, Ganam L G, Fernandez-Salguero P M, Gonzalez F J, Jefcoate C R. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111:3311–3322. - PubMed
-
- Andreola F, Fernandez-Salguero P M, Chiantore M V, Petkovich M P, Gonzalez F J, De Luca L M. Aryl hydrocarbon receptor knock-out mice (Ahr−/−) exhibit liver retinoid accumulation and reduced retinoid acid metabolism. Cancer Res. 1997;57:2835–2838. - PubMed
-
- Austin A J, Crabtree G R, Schreiber S L. Proximity versus allostery: the role of regulated protein dimerization in biology. Chem Biol. 1994;1:131–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
