Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes
- PMID: 11238933
- PMCID: PMC86790
- DOI: 10.1128/MCB.21.6.1973-1985.2001
Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes
Abstract
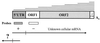
In the human genome, retrotranspositionally competent long interspersed nuclear elements (L1Hs) are involved in the generation of processed pseudogenes and mobilization of unrelated sequences into existing genes. Transcription of each L1Hs is initiated from its internal promoter but may also be driven from the promoters of adjacent cellular genes. Here I show that a hitherto unknown L1Hs antisense promoter (ASP) drives the transcription of adjacent genes. The ASP is located in the L1Hs 5' untranslated region (5'UTR) and works in the opposite direction. Fifteen cDNAs, isolated from a human NTera2D1 cDNA library by a differential screening method, contained L1Hs 5'UTRs spliced to the sequences of known genes or non-proteincoding sequences. Four of these chimeric transcripts, selected for detailed analysis, were detected in total RNA of different cell lines. Their abundance accounted for roughly 1 to 500% of the transcripts of four known genes, suggesting a large variation in the efficiency of L1Hs ASP-driven transcription. ASP-directed transcription was also revealed from expressed sequence tag sequences and confirmed by using an RNA dot blot analysis. Nine of the 15 randomly selected genomic L1Hs 5'UTRs had ASP activities about 7- to 50-fold higher than background in transient transfection assays. ASP was assigned to the L1Hs 5'UTR between nucleotides 400 to 600 by deletion and mutation analysis. These results indicate that many L1Hs contain active ASPs which are capable of interfering with normal gene expression, and this type of transcriptional control may be widespread.
Figures







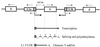
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
