Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression
- PMID: 11238953
- PMCID: PMC86854
- DOI: 10.1128/MCB.21.6.2203-2212.2001
Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression
Abstract
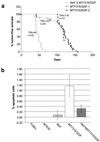
Activation of Akt by the phosphatidylinositol 3'-OH kinase (PI3K) results in the inhibition of proapoptotic signals and the promotion of survival signals (L. P. Kane et al., Curr. Biol. 9:601-604, 1999; G. J. Kops et al., Nature 398:630-634, 1999). Evidence supporting the importance of the PI3K/Akt signaling pathway in tumorigenesis stems from experiments with transgenic mice bearing polyomavirus middle T antigen under the control of the mouse mammary tumor virus long terminal repeat promoter. Mammary epithelium-specific expression of polyomavirus middle T antigen results in the rapid development of multifocal metastatic mammary tumors, whereas transgenic mice expressing a mutant middle T antigen decoupled from the phosphatidylinositol 3'-OH kinase (MTY315/322F) develop extensive mammary gland hyperplasias that are highly apoptotic. To directly assess the role of Akt in mammary epithelial development and tumorigenesis, we generated transgenic mice expressing constitutively active Akt (HAPKB308D473D or Akt-DD). Although expression of Akt-DD interferes with normal mammary gland involution, tumors were not observed in these strains. However, coexpression of Akt-DD with MTY315/322F resulted in a dramatic acceleration of mammary tumorigenesis correlated with reduced apoptotic cell death. Furthermore, coexpression of Akt-DD with MTY315/322F resulted in phosphorylation of the FKHR forkhead transcription factor and translational upregulation of cyclin D1 levels. Importantly, we did not observe an associated restoration of wild-type metastasis levels in the bitransgenic strain. Taken together these observations indicate that activation of Akt can contribute to tumor progression by providing an important cell survival signal but does not promote metastatic progression.
Figures





References
-
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. - PubMed
-
- Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
