Disruption of the mouse mu-calpain gene reveals an essential role in platelet function
- PMID: 11238954
- PMCID: PMC86855
- DOI: 10.1128/MCB.21.6.2213-2220.2001
Disruption of the mouse mu-calpain gene reveals an essential role in platelet function
Abstract
Conventional calpains are ubiquitous calcium-regulated cysteine proteases that have been implicated in cytoskeletal organization, cell proliferation, apoptosis, cell motility, and hemostasis. There are two forms of conventional calpains: the mu-calpain, or calpain I, which requires micromolar calcium for half-maximal activation, and the m-calpain, or calpain II, which functions at millimolar calcium concentrations. We evaluated the functional role of the 80-kDa catalytic subunit of mu-calpain by genetic inactivation using homologous recombination in embryonic stem cells. The mu-calpain-deficient mice are viable and fertile. The complete deficiency of mu-calpain causes significant reduction in platelet aggregation and clot retraction but surprisingly the mutant mice display normal bleeding times. No detectable differences were observed in the cleavage pattern and kinetics of calpain substrates such as the beta3 subunit of alphaIIbbeta3 integrin, talin, and ABP-280 (filamin). However, mu-calpain null platelets exhibit impaired tyrosine phosphorylation of several proteins including the beta3 subunit of alphaIIbbeta3 integrin, correlating with the agonist-induced reduction in platelet aggregation. These results provide the first direct evidence that mu-calpain is essential for normal platelet function, not by affecting the cleavage of cytoskeletal proteins but by potentially regulating the state of tyrosine phosphorylation of the platelet proteins.
Figures
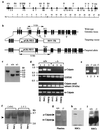


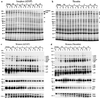
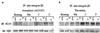
References
-
- Calderwood D A, Zent R, Grant R, Rees D J, Hynes R O, Ginsberg M H. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. - PubMed
-
- Cichowski K, McCormick F, Brugge J S. p21rasGAP association with Fyn, Lyn, and Yes in thrombin-activated platelets. J Biol Chem. 1992;267:5025–5028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
