Eukaryotic ribonuclease P: increased complexity to cope with the nuclear pre-tRNA pathway
- PMID: 11241345
- PMCID: PMC3758117
- DOI: 10.1002/1097-4652(200104)187:1<11::AID-JCP1055>3.0.CO;2-K
Eukaryotic ribonuclease P: increased complexity to cope with the nuclear pre-tRNA pathway
Abstract
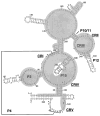
Ribonuclease P is an ancient enzyme that cleaves pre-tRNAs to generate mature 5' ends. It contains an essential RNA subunit in Bacteria, Archaea, and Eukarya, but the degree to which the RNA subunit relies on proteins to supplement catalysis is highly variable. The eukaryotic nuclear holoenzyme has recently been found to contain almost twenty times the protein content of the bacterial enzymes, in addition to having split into at least two related enzymes with distinct substrate specificity. In this review, recent progress in understanding the molecular architecture and functions of nuclear forms of RNase P will be considered.
Copyright 2001 Wiley-Liss, Inc.
Figures

References
-
- Akaboshi E, Guerrier-Takada C, Altman S. Veal heart ribonuclease P has an essential RNA component. Biochem Biophys Res Commun. 1980;96:831–837. - PubMed
-
- Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 1994;8:3021–3031. - PubMed
-
- Altman S, Wesolowski D, Puranam RS. Nucleotide sequences of the RNA subunit of RNase P from several mammals. Genomics. 1993;18:418–422. - PubMed
-
- Arends S, Schon A. Partial purification and characterization of nuclear ribonuclease P from wheat. Eur J Biochem. 1997;244:635–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

