Identification of Mutator insertional mutants of starch-branching enzyme 2a in corn
- PMID: 11244119
- PMCID: PMC65618
- DOI: 10.1104/pp.125.3.1396
Identification of Mutator insertional mutants of starch-branching enzyme 2a in corn
Abstract
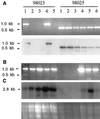
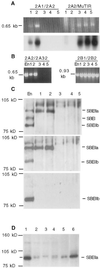
Starch-branching enzymes (SBE) break the alpha-1,4 linkage of starch, re-attaching the chain to a glucan chain by an alpha-1,6 bond, altering starch structure. SBEs also facilitate starch accumulation by increasing the number of non-reducing ends on the growing chain. In maize (Zea mays), three isoforms of SBE have been identified. To examine the function of the SBEIIa isoform, a reverse genetics polymerase chain reaction-based screen was used to identify a mutant line segregating for a Mutator transposon within Sbe2a. To locate the insertion within the second exon of Sbe2a, the genomic sequence of Sbe2a containing the promoter and 5' end was isolated and sequenced. Plants homozygous for sbe2a::Mu have undetectable levels of Sbe2a transcripts and SBEIIa in their leaves. Characterization of leaf starch from sbe2a::Mu mutants shows reduced branching similar to yet more extreme than that seen in kernels lacking SBEIIb activity. Characterization of endosperm starch from sbe2a::Mu mutants shows branching that is indistinguishable from wild-type controls. These mutant plants have a visible phenotype resembling accelerated senescence, which was correlated with the Mutator insertion within Sbe2a. This correlation suggests a specific role for SBEIIa in leaves, which may be necessary for normal plant development.
Figures




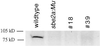
References
-
- Baba T, Kimura K, Mizuno K, Etoh H, Ishida Y, Shida O, Arai Y. Sequence conservation of the catalytic regions of amylolytic enzymes in maize branching enzyme-I. Biochem Biophys Res Commun. 1991;181:87–94. - PubMed
-
- Boyer CD, Liu K-C. The interaction of endosperm genotype and genetic background: I. Differences in chromatographic profiles of starches from nonmutant and mutant endosperms. Staerke. 1985;37:73–79.
-
- Boyer CD, Preiss J. Multiple forms of (1,4)-α-d-glucan, (1,4)-α-d-glucan-6-glycosyl transferase from developing Zea mays L. kernels. Carbohydr Res. 1978;61:321–334.
-
- Chandler VL, Hardeman KJ. The Mu elements of Zea mays. Adv Genet. 1992;30:77–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

