A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity
- PMID: 11244130
- PMCID: PMC65629
- DOI: 10.1104/pp.125.3.1517
A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity
Abstract
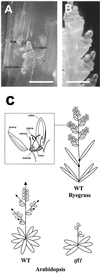
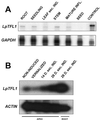
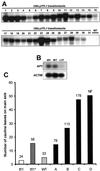
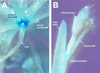
Control of flowering and the regulation of plant architecture have been thoroughly investigated in a number of well-studied dicot plants such as Arabidopsis, Antirrhinum, and tobacco. However, in many important monocot seed crops, molecular information on plant reproduction is still limited. To investigate the regulation of meristem identity and the control of floral transition in perennial ryegrass (Lolium perenne) we isolated a ryegrass TERMINAL FLOWER1-like gene, LpTFL1, and characterized it for its function in ryegrass flower development. Perennial ryegrass requires a cold treatment of at least 12 weeks to induce flowering. During this period a decrease in LpTFL1 message was detected in the ryegrass apex. However, upon subsequent induction with elevated temperatures and long-day photoperiods, LpTFL1 message levels increased and reached a maximum when the ryegrass apex has formed visible spikelets. Arabidopsis plants overexpressing LpTFL1 were significantly delayed in flowering and exhibited dramatic changes in architecture such as extensive lateral branching, increased growth of all vegetative organs, and a highly increased trichome production. Furthermore, overexpression of LpTFL1 was able to complement the phenotype of the severe tfl1-14 mutant of Arabidopsis. Analysis of the LpTFL1 promoter fused to the UidA gene in Arabidopsis revealed that the promoter is active in axillary meristems, but not the apical meristem. Therefore, we suggest that LpTFL1 is a repressor of flowering and a controller of axillary meristem identity in ryegrass.
Figures


Similar articles
-
Isolation and characterization of cold-regulated transcriptional activator LpCBF3 gene from perennial ryegrass (Lolium perenne L.).Mol Genet Genomics. 2008 Jun;279(6):585-94. doi: 10.1007/s00438-008-0335-4. Epub 2008 Mar 20. Mol Genet Genomics. 2008. PMID: 18351391
-
Nucleotide diversity and linkage disequilibrium of nine genes with putative effects on flowering time in perennial ryegrass (Lolium perenne L.).Plant Sci. 2011 Feb;180(2):228-37. doi: 10.1016/j.plantsci.2010.08.015. Epub 2010 Aug 27. Plant Sci. 2011. PMID: 21421365
-
Photoperiodic regulation of flowering in perennial ryegrass involving a CONSTANS -like homolog.Plant Mol Biol. 2004 Sep;56(2):159-69. doi: 10.1007/s11103-004-2647-z. Plant Mol Biol. 2004. PMID: 15604735
-
Turning Meristems into Fortresses.Trends Plant Sci. 2019 May;24(5):431-442. doi: 10.1016/j.tplants.2019.02.004. Epub 2019 Mar 7. Trends Plant Sci. 2019. PMID: 30853243 Review.
-
To be, or not to be, a flower--control of floral meristem identity.Trends Genet. 1998 Jan;14(1):26-32. doi: 10.1016/S0168-9525(97)01309-7. Trends Genet. 1998. PMID: 9448463 Review.
Cited by
-
Knock-out of TERMINAL FLOWER 1 genes altered flowering time and plant architecture in Brassica napus.BMC Genet. 2020 May 19;21(1):52. doi: 10.1186/s12863-020-00857-z. BMC Genet. 2020. PMID: 32429836 Free PMC article.
-
BdRCN4, a Brachypodium distachyon TFL1 homologue, is involved in regulation of apical meristem fate.Plant Mol Biol. 2024 Jun 28;114(4):81. doi: 10.1007/s11103-024-01467-4. Plant Mol Biol. 2024. PMID: 38940986
-
Isolation of a CENTRORADIALIS/TERMINAL FLOWER1 homolog in saffron (Crocus sativus L.): characterization and expression analysis.Mol Biol Rep. 2012 Aug;39(8):7899-910. doi: 10.1007/s11033-012-1634-8. Epub 2012 Apr 26. Mol Biol Rep. 2012. PMID: 22535321
-
QTL mapping of vernalization response in perennial ryegrass (Lolium perenne L.) reveals co-location with an orthologue of wheat VRN1.Theor Appl Genet. 2005 Feb;110(3):527-36. doi: 10.1007/s00122-004-1865-8. Epub 2004 Dec 24. Theor Appl Genet. 2005. PMID: 15619078
-
CENTRORADIALIS Interacts with FLOWERING LOCUS T-Like Genes to Control Floret Development and Grain Number.Plant Physiol. 2019 Jun;180(2):1013-1030. doi: 10.1104/pp.18.01454. Epub 2019 Apr 19. Plant Physiol. 2019. PMID: 31004004 Free PMC article.
References
-
- Alvarez J, Guli CL, Yu X, Smyth DR. Terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2:103–116.
-
- Banfield MJ, Brady RL. The structure of Antirrhinum Centroradialisprotein (CEN) suggests a role as a kinase regulator. J Mol Biol. 2000;297:1159–1170. - PubMed
-
- Barnard C. Floral histogenesis in the monocotyledons: I. The Granineae. Aust J Bot. 1957;5:115–128.
-
- Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen E. Control of inflorescence architecture in Antirrhinum. Nature. 1996;376:791–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

