Subunit-dependent modulation of neuronal nicotinic receptors by zinc
- PMID: 11245669
- PMCID: PMC6762592
- DOI: 10.1523/JNEUROSCI.21-06-01848.2001
Subunit-dependent modulation of neuronal nicotinic receptors by zinc
Abstract
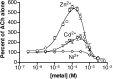
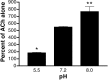
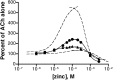
We examined the effect of zinc on rat neuronal nicotinic acetylcholine receptors (nAChRs) expressed in Xenopus oocytes as simple heteromers of alpha2, alpha3, or alpha4 and beta2 or beta4. Coapplication of zinc with low concentrations of acetylcholine (</=EC(10)) resulted in differential effects depending on receptor subunit composition. The alpha2beta2, alpha2beta4, alpha3beta4, alpha4beta2, and alpha4beta4 receptors exhibited biphasic modulation by zinc, with potentiation of the acetylcholine response occurring at 1-100 micrometer zinc and inhibition occurring at higher zinc concentrations. In contrast, alpha3beta2 receptors were only inhibited by zinc (IC(50) = 97 +/- 16 micrometer). The greatest potentiating effect of zinc was seen with alpha4beta4 receptors that were potentiated to 560 +/- 17% of the response to ACh alone, with an EC(50) of 22 +/- 4 micrometer zinc. Cadmium, but not nickel, was also able to potentiate alpha4beta4 receptors. Both zinc potentiation of alpha4beta4 receptors and zinc inhibition of alpha3beta2 receptors were voltage independent. The sensitivity of zinc potentiation of alpha4beta4 to diethylpyrocarbonate treatment and alterations in pH suggested the involvement of histidine residues. Zinc continued to inhibit alpha4beta4 and alpha3beta2 after diethylpyrocarbonate treatment. Application of a potentiating zinc concentration increased the response of alpha4beta2 and alpha4beta4 receptors to saturating ACh concentrations. The rate of Ach-induced desensitization of these receptors was unaffected by zinc. Our results reveal zinc potentiation as a new mode of neuronal nAChR modulation.
Figures






Similar articles
-
Competitive potentiation of acetylcholine effects on neuronal nicotinic receptors by acetylcholinesterase-inhibiting drugs.J Neurochem. 2000 Dec;75(6):2492-500. doi: 10.1046/j.1471-4159.2000.0752492.x. J Neurochem. 2000. PMID: 11080202
-
Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects.Mol Pharmacol. 1997 Nov;52(5):886-95. doi: 10.1124/mol.52.5.886. Mol Pharmacol. 1997. PMID: 9351980
-
Interaction of bupropion and zinc with neuronal nicotinic acetylcholine receptors.Neuropharmacology. 2011 Dec;61(8):1202-9. doi: 10.1016/j.neuropharm.2011.07.009. Epub 2011 Jul 26. Neuropharmacology. 2011. PMID: 21791218
-
Effects of pyridine ring substitutions on affinity, efficacy, and subtype selectivity of neuronal nicotinic receptor agonist epibatidine.J Pharmacol Exp Ther. 2002 Sep;302(3):1246-52. doi: 10.1124/jpet.102.035899. J Pharmacol Exp Ther. 2002. PMID: 12183686
-
Zinc potentiates neuronal nicotinic receptors by increasing burst duration.J Neurophysiol. 2008 Feb;99(2):999-1007. doi: 10.1152/jn.01040.2007. Epub 2007 Dec 19. J Neurophysiol. 2008. PMID: 18094103
Cited by
-
Using C. elegans to decipher the cellular and molecular mechanisms underlying neurodevelopmental disorders.Mol Neurobiol. 2013 Dec;48(3):465-89. doi: 10.1007/s12035-013-8434-6. Epub 2013 Mar 14. Mol Neurobiol. 2013. PMID: 23494747 Review.
-
Human α3β4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells.PLoS One. 2010 Oct 26;5(10):e13611. doi: 10.1371/journal.pone.0013611. PLoS One. 2010. PMID: 21049012 Free PMC article.
-
Non-agonist-binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the alpha4beta2 nicotinic receptor: an alpha4-alpha4 interface is required for Zn2+ potentiation.J Neurosci. 2008 Jul 2;28(27):6884-94. doi: 10.1523/JNEUROSCI.1228-08.2008. J Neurosci. 2008. PMID: 18596163 Free PMC article.
-
Contribution of extracellular negatively charged residues to ATP action and zinc modulation of rat P2X2 receptors.J Neurochem. 2008 May;105(4):1264-75. doi: 10.1111/j.1471-4159.2008.05228.x. Epub 2008 Jan 14. J Neurochem. 2008. PMID: 18194442 Free PMC article.
-
Nicotine Increases Spontaneous Glutamate Release in the Rostromedial Tegmental Nucleus.Front Neurosci. 2021 Jan 13;14:604583. doi: 10.3389/fnins.2020.604583. eCollection 2020. Front Neurosci. 2021. PMID: 33519359 Free PMC article.
References
-
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–11198. - PubMed
-
- Assaf SY, Chung S-H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by zinc. Mol Pharmacol. 1994;46:1156–1159. - PubMed
-
- Cachelin AB, Rust G. Unusual pharmacology of (+)-tubocurarine with rat neuronal nicotinic acetylcholine receptors containing β4 subunits. Mol Pharmacol. 1994;46:1168–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
