Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain
- PMID: 11245672
- PMCID: PMC6762628
- DOI: 10.1523/JNEUROSCI.21-06-01876.2001
Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain
Abstract
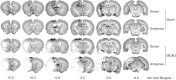
Transient focal cerebral ischemia leads to extensive neuronal damage in cerebral cortex and striatum. Normal functioning of glutamate transporters clears the synaptically released glutamate to prevent excitotoxic neuronal death. This study evaluated the functional role of the glial (GLT-1) and neuronal (EAAC1) glutamate transporters in mediating ischemic neuronal damage after transient middle cerebral artery occlusion (MCAO). Transient MCAO in rats infused with GLT-1 antisense oligodeoxynucleotides (ODNs) led to increased infarct volume (45 +/- 8%; p < 0.05), worsened neurological status, and increased mortality rate, compared with GLT-1 sense/random ODN-infused controls. Transient MCAO in rats infused with EAAC1 antisense ODNs had no significant effect on any of these parameters. This study suggests that GLT-1, but not EAAC1, knockdown exacerbates the neuronal death and thus neurological deficit after stroke.
Figures





Similar articles
-
Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain.Neurochem Res. 2001 May;26(5):497-502. doi: 10.1023/a:1010956711295. Neurochem Res. 2001. PMID: 11513475
-
Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain.Eur J Neurosci. 2001 Jan;13(1):119-28. Eur J Neurosci. 2001. PMID: 11135010
-
Altered expression of the glutamate transporter EAAC1 in neurons and immature oligodendrocytes after transient forebrain ischemia.J Cereb Blood Flow Metab. 2000 Apr;20(4):678-87. doi: 10.1097/00004647-200004000-00005. J Cereb Blood Flow Metab. 2000. PMID: 10779012
-
Glutamate uptake.Prog Neurobiol. 2001 Sep;65(1):1-105. doi: 10.1016/s0301-0082(00)00067-8. Prog Neurobiol. 2001. PMID: 11369436 Review.
-
Role of Excitatory Amino Acid Carrier 1 (EAAC1) in Neuronal Death and Neurogenesis After Ischemic Stroke.Int J Mol Sci. 2020 Aug 7;21(16):5676. doi: 10.3390/ijms21165676. Int J Mol Sci. 2020. PMID: 32784778 Free PMC article. Review.
Cited by
-
Electroacupuncture preconditioning-induced neuroprotection may be mediated by glutamate transporter type 2.Neurochem Int. 2013 Oct;63(4):302-8. doi: 10.1016/j.neuint.2013.06.017. Epub 2013 Jul 4. Neurochem Int. 2013. PMID: 23831620 Free PMC article.
-
Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses.J Neurosci. 2003 Jun 1;23(11):4775-84. doi: 10.1523/JNEUROSCI.23-11-04775.2003. J Neurosci. 2003. PMID: 12805317 Free PMC article.
-
Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1.J Neurosci. 2003 Aug 6;23(18):7176-82. doi: 10.1523/JNEUROSCI.23-18-07176.2003. J Neurosci. 2003. PMID: 12904478 Free PMC article.
-
Pre-ischemic Treatment with Ampicillin Reduces Neuronal Damage in the Mouse Hippocampus and Neostriatum after Transient Forebrain Ischemia.Korean J Physiol Pharmacol. 2008 Dec;12(6):287-91. doi: 10.4196/kjpp.2008.12.6.287. Epub 2008 Dec 31. Korean J Physiol Pharmacol. 2008. PMID: 19967069 Free PMC article.
-
Glial cells in the center of future ischemic stroke treatments.Neural Regen Res. 2022 Dec;17(12):2659-2660. doi: 10.4103/1673-5374.339480. Neural Regen Res. 2022. PMID: 35662199 Free PMC article. No abstract available.
References
-
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. - PubMed
-
- Chaudhry FA, Lehre KP, Campangne ML, Otterson OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma lemma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:717–720. - PubMed
-
- Chen W, Aoki C, Gruber C, Hadley R, Wang G, Blitzblau R, Volpe JJ, Irwin N, Rosenberg PA. Molecular cloning, functional characterization, and neuronal localization of a variant form of the glutamate transporter GLT-1. Soc Neurosci Abstr. 2000;26:539.15.
-
- Coccia C, Ganel R, Rothstein JD. GDNF induces an increase in EAAT2 (GLT-1) expression. Soc Neurosci Abstr. 1999;25:170.2.
-
- Danbolt NC, Chaudhry FA, Dehnes Y, Lehre KP, Levy LM, Ullensvang K, Storm-Mathisen J. Properties and localization of glutamate transporters. Prog Brain Res. 1998;116:23–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
