Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons
- PMID: 11245684
- PMCID: PMC6762608
- DOI: 10.1523/JNEUROSCI.21-06-02001.2001
Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons
Abstract
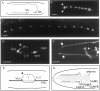
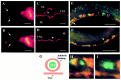
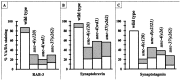
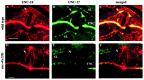
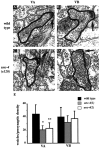
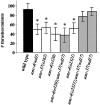
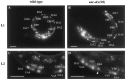
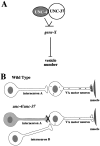
Motor neuron function depends on neurotransmitter release from synaptic vesicles (SVs). Here we show that the UNC-4 homeoprotein and its transcriptional corepressor protein UNC-37 regulate SV protein levels in specific Caenorhabditis elegans motor neurons. UNC-4 is expressed in four classes (DA, VA, VC, and SAB) of cholinergic motor neurons. Antibody staining reveals that five different vesicular proteins (UNC-17, choline acetyltransferase, Synaptotagmin, Synaptobrevin, and RAB-3) are substantially reduced in unc-4 and unc-37 mutants in these cells; nonvesicular neuronal proteins (Syntaxin, UNC-18, and UNC-11) are not affected, however. Ultrastructural analysis of VA motor neurons in the mutant unc-4(e120) confirms that SV number in the presynaptic zone is reduced ( approximately 40%) whereas axonal diameter and synaptic morphology are not visibly altered. Because the UNC-4-UNC-37 complex has been shown to mediate transcriptional repression, we propose that these effects are performed via an intermediate gene. Our results are consistent with a model in which this unc-4 target gene ("gene-x") functions at a post-transcriptional level as a negative regulator of SV biogenesis or stability. Experiments with a temperature-sensitive unc-4 mutant show that the adult level of SV proteins strictly depends on unc-4 function during a critical period of motor neuron differentiation. unc-4 activity during this sensitive larval stage is also required for the creation of proper synaptic inputs to VA motor neurons. The temporal correlation of these events may mean that a common unc-4-dependent mechanism controls both the specificity of synaptic inputs as well as the strength of synaptic outputs for these motor neurons.
Figures

References
-
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. - PubMed
-
- Alfonso A, Grundahl K, McManus JR, Asbury JM, Rand JB. Alternative splicing leads to two cholinergic proteins in Caenorhabditis elegans. J Mol Biol. 1994;241:627–630. - PubMed
-
- Bergmann M, Lahr G, Mayerhofer A, Gratzl M. Expression of synaptophysin during the prenatal development of the rat spinal cord: correlation with basic differentiation processes of neurons. Neuroscience. 1991;42:569–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
