Regulation of microglial development: a novel role for thyroid hormone
- PMID: 11245686
- PMCID: PMC6762591
- DOI: 10.1523/JNEUROSCI.21-06-02028.2001
Regulation of microglial development: a novel role for thyroid hormone
Abstract
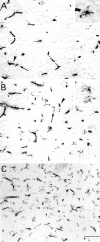
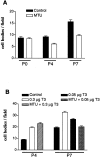
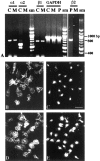
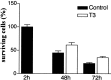
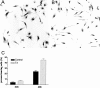
The postnatal development of rat microglia is marked by an important increase in the number of microglial cells and the growth of their ramified processes. We studied the role of thyroid hormone in microglial development. The distribution and morphology of microglial cells stained with isolectin B4 or monoclonal antibody ED1 were analyzed in cortical and subcortical forebrain regions of developing rats rendered hypothyroid by prenatal and postnatal treatment with methyl-thiouracil. Microglial processes were markedly less abundant in hypothyroid pups than in age-matched normal animals, from postnatal day 4 up to the end of the third postnatal week of life. A delay in process extension and a decrease in the density of microglial cell bodies, as shown by cell counts in the developing cingulate cortex of normal and hypothyroid animals, were responsible for these differences. Conversely, neonatal rat hyperthyroidism, induced by daily injections of 3,5,3'-triiodothyronine (T3), accelerated the extension of microglial processes and increased the density of cortical microglial cell bodies above physiological levels during the first postnatal week of life. Reverse transcription-PCR and immunological analyses indicated that cultured cortical ameboid microglial cells expressed the alpha1 and beta1 isoforms of nuclear thyroid hormone receptors. Consistent with the trophic and morphogenetic effects of thyroid hormone observed in situ, T3 favored the survival of cultured purified microglial cells and the growth of their processes. These results demonstrate that thyroid hormone promotes the growth and morphological differentiation of microglia during development.
Figures



References
-
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors originating from the yolk sac and which proliferate in the brain. Dev Brain Res. 1999;117:145–152. - PubMed
-
- Antel JP, Owens T. Immune regulation and CNS autoimmune disease. J Neuroimmunol. 1999;100:181–189. - PubMed
-
- Arpin C, Pihlgren M, Fraichard A, Aubert D, Samarut J, Chassande O, Marvel J. Effects of T3R alpha 1 and T3R alpha 2 gene deletion on T and B lymphocyte development. J Immunol. 2000;164:152–160. - PubMed
-
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Dev Brain Res. 1991;58:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
