Localized injury in cardiomyocyte network: a new experimental model of ischemia-reperfusion arrhythmias
- PMID: 11247808
- PMCID: PMC3019580
- DOI: 10.1152/ajpheart.2001.280.4.H1905
Localized injury in cardiomyocyte network: a new experimental model of ischemia-reperfusion arrhythmias
Abstract
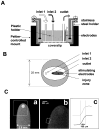
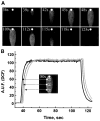
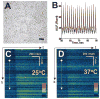
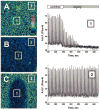
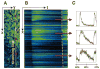
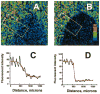
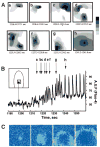
We developed a new experimental approach to study the effects of local injury in a multicellular preparation and tested the ability of the method to induce reperfusion arrhythmias in cardiomyocyte monolayers. A small region of injury was created using geometrically defined flows of control and ischemia-like solutions. Calcium transients were acquired simultaneously from injured, control, and border zone cells using fluo 4. Superfusion with the injury solution rapidly diminished the amplitude of calcium transients within the injury zone, followed by cessation of cell beating. Reperfusion caused an immediate tachyarrhythmic response in approximately 17% of experiments, with a wave front propagating from a single cell or small cell cluster within the former injury zone. Inclusion of a gap junction uncoupler (1 mM heptanol) in the injury solution narrowed the functional border and sharply increased the number of ectopic foci and the incidence of reperfusion arrhythmias. The model holds a potential to reveal both micro- and macroscopic features of propagation, conduction, and cell coupling in the normal and diseased myocardium and to serve as a new tool to test antiarrhythmic protocols in vitro.
Figures

References
-
- Abramovich-Sivan S, Akselrod S. Simulation of atrial activity by a phase response curve based model of a two-dimensional pacemaker cells array: the transition from a normal activation pattern to atrial fibrillation. Biol Cybern. 1999;80:141–153. - PubMed
-
- Arutunian A, Webster D, Swift L, Sarvazyan N. A new experimental model to emulate ischemia-reperfusion arrhythmias in monolayers of cultured cardiomyocytes (Abstract) Biophys J. 2000;78:454A.
-
- Atsma DE, Bastiaanse EM, Ince C, van der Laarse A. A novel two-compartment culture dish allows microscopic evaluation of two different treatments in one cell culture simultaneously. Influence of external pH on Na+/Ca2+ exchanger activity in cultured rat cardiomyocytes. Pflügers Arch. 1994;428:296–299. - PubMed
-
- Barrigon S, Wang SY, Ji X, Langer GA. Characterization of the calcium overload in cultured neonatal rat cardiomyocytes under metabolic inhibition. J Mol Cell Cardiol. 1996;28:1329–1337. - PubMed
-
- Boersma L, Brugada J, Kirchhof C, Allessie M. Entrainment of reentrant ventricular tachycardia in anisotropic rings of rabbit myocardium. Mechanisms of termination, changes in morphology, and acceleration. Circulation. 1993;88:1852–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

