Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography
- PMID: 11248072
- PMCID: PMC30647
- DOI: 10.1073/pnas.051604498
Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography
Abstract
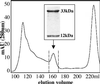
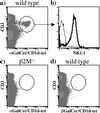
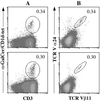
CD1 molecules are specialized in presenting lipids to T lymphocytes, but identification and isolation of CD1-restricted lipid specific T cells has been hampered by the lack of reliable and sensitive techniques. We here report the construction of CD1d-glycolipid tetramers from fully denatured human CD1d molecules by using the technique of oxidative refolding chromatography. We demonstrate that chaperone- and foldase-assisted refolding of denatured CD1d molecules and beta(2)-microglobulin in the presence of synthetic lipids is a rapid method for the generation of functional and specific CD1d tetramers, which unlike previously published protocols ensures isolation of CD1d tetramers loaded with a single lipid species. The use of human CD1d-alpha-galactosylceramide tetramers for ex vivo staining of peripheral blood lymphocytes and intrahepatic T cells from patients with viral liver cirrhosis allowed for the first time simultaneous analysis of frequency and specificity of natural killer T cells in human clinical samples. Application of this protocol to other members of the CD1 family will provide powerful tools to investigate lipid-specific T cell immune responses in health and in disease.
Figures


Comment in
-
Right on target: novel approaches for the direct visualization of CD1-specific T cell responses.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):2950-2. doi: 10.1073/pnas.061032398. Proc Natl Acad Sci U S A. 2001. PMID: 11248010 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

