Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo
- PMID: 11248078
- PMCID: PMC30653
- DOI: 10.1073/pnas.041614798
Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo
Abstract
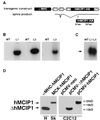
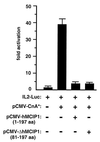
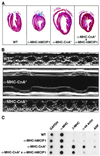
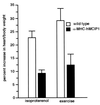
Signaling events controlled by calcineurin promote cardiac hypertrophy, but the degree to which such pathways are required to transduce the effects of various hypertrophic stimuli remains uncertain. In particular, the administration of immunosuppressive drugs that inhibit calcineurin has inconsistent effects in blocking cardiac hypertrophy in various animal models. As an alternative approach to inhibiting calcineurin in the hearts of intact animals, transgenic mice were engineered to overexpress a human cDNA encoding the calcineurin-binding protein, myocyte-enriched calcineurin-interacting protein-1 (hMCIP1) under control of the cardiac-specific, alpha-myosin heavy chain promoter (alpha-MHC). In unstressed mice, forced expression of hMCIP1 resulted in a 5-10% decline in cardiac mass relative to wild-type littermates, but otherwise produced no apparent structural or functional abnormalities. However, cardiac-specific expression of hMCIP1 inhibited cardiac hypertrophy, reinduction of fetal gene expression, and progression to dilated cardiomyopathy that otherwise result from expression of a constitutively active form of calcineurin. Expression of the hMCIP1 transgene also inhibited hypertrophic responses to beta-adrenergic receptor stimulation or exercise training. These results demonstrate that levels of hMCIP1 producing no apparent deleterious effects in cells of the normal heart are sufficient to inhibit several forms of cardiac hypertrophy, and suggest an important role for calcineurin signaling in diverse forms of cardiac hypertrophy. The future development of measures to increase expression or activity of MCIP proteins selectively within the heart may have clinical value for prevention of heart failure.
Figures
Comment in
-
Calcineurin inhibition and cardiac hypertrophy: a matter of balance.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):2947-9. doi: 10.1073/pnas.051033698. Proc Natl Acad Sci U S A. 2001. PMID: 11248009 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

