Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines
- PMID: 11248104
- PMCID: PMC30679
- DOI: 10.1073/pnas.051628698
Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines
Abstract
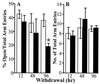
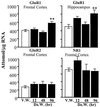
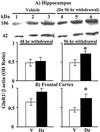
Protracted administration of diazepam elicits tolerance, whereas discontinuation of treatment results in signs of dependence. Tolerance to the anticonvulsant action of diazepam is present in an early phase (6, 24, and 36 h) but disappears in a late phase (72-96 h) of withdrawal. In contrast, signs of dependence such as decrease in open-arm entries on an elevated plus-maze and increased susceptibility to pentylenetetrazol-induced seizures were apparent 96 h (but not 12, 24, or 48 h) after diazepam withdrawal. During the first 72 h of withdrawal, tolerance is associated with changes in the expression of GABA(A) (gamma-aminobutyric acid type A) receptor subunits (decrease in gamma(2) and alpha(1); increase in alpha(5)) and with an increase of mRNA expression of the most abundant form of glutamic acid decarboxylase (GAD), GAD(67). In contrast, dl-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor GluR1 subunit mRNA and cognate protein, which are normal during the early phase of diazepam withdrawal, increase by approximately 30% in cortex and hippocampus in association with the appearance of signs of dependence 96 h after diazepam withdrawal. Immunohistochemical studies of GluR1 subunit expression with gold-immunolabeling technique reveal that the increase of GluR1 subunit protein is localized to layer V pyramidal neurons and their apical dendrites in the cortex, and to pyramidal neurons and in their dendritic fields in hippocampus. The results suggest an involvement of GABA-mediated processes in the development and maintenance of tolerance to diazepam, whereas excitatory amino acid-related processes (presumably via AMPA receptors) may be involved in the expression of signs of dependence after withdrawal.
Figures





Similar articles
-
Modifications of gamma-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam.Mol Pharmacol. 1996 May;49(5):822-31. Mol Pharmacol. 1996. PMID: 8622632
-
Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase.Neuroscience. 2005;132(2):399-407. doi: 10.1016/j.neuroscience.2005.01.005. Neuroscience. 2005. PMID: 15802192
-
alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionate receptor autoradiography in mouse brain after single and repeated withdrawal from diazepam.Eur J Neurosci. 2005 Feb;21(4):1045-56. doi: 10.1111/j.1460-9568.2005.03902.x. Eur J Neurosci. 2005. PMID: 15787709
-
GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon.Psychopharmacology (Berl). 2005 Jul;180(2):191-205. doi: 10.1007/s00213-005-2212-8. Epub 2005 Apr 28. Psychopharmacology (Berl). 2005. PMID: 15864560 Review.
-
GABAA receptors and benzodiazepines: a role for dendritic resident subunit mRNAs.Neuropharmacology. 2002 Nov;43(6):925-37. doi: 10.1016/s0028-3908(02)00199-5. Neuropharmacology. 2002. PMID: 12423662 Review.
Cited by
-
Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABA(A) Receptor Modulators?Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. Epub 2012 Mar 29. Adv Pharmacol Sci. 2012. PMID: 22536226 Free PMC article.
-
Regulation of Ca²⁺/calmodulin-dependent protein kinase II signaling within hippocampal glutamatergic postsynapses during flurazepam withdrawal.Neural Plast. 2012;2012:405926. doi: 10.1155/2012/405926. Epub 2012 Jul 5. Neural Plast. 2012. PMID: 22830051 Free PMC article.
-
Adaptive changes in the rat hippocampal glutamatergic neurotransmission are observed during long-term treatment with lorazepam.Psychopharmacology (Berl). 2003 Mar;166(2):163-7. doi: 10.1007/s00213-002-1373-y. Epub 2003 Jan 24. Psychopharmacology (Berl). 2003. PMID: 12545333
-
Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal.J Comp Neurol. 2008 Dec 20;511(6):832-46. doi: 10.1002/cne.21866. J Comp Neurol. 2008. PMID: 18924138 Free PMC article.
-
Prolonged Alprazolam Treatment Alters Components of Glutamatergic Neurotransmission in the Hippocampus of Male Wistar Rats-The Neuroadaptive Changes following Long-Term Benzodiazepine (Mis)Use.Pharmaceuticals (Basel). 2023 Feb 21;16(3):331. doi: 10.3390/ph16030331. Pharmaceuticals (Basel). 2023. PMID: 36986431 Free PMC article.
References
-
- Woods J H, Katz L A, Winger G. Pharmacol Rev. 1992;44:151–347. - PubMed
-
- Costa E, Auta J, Guidotti A. In: Handbook of Experimental Pharmacology: Pharmacology of Inhibitory Amino Acid Transmitters. Möhler H, editor. Vol. 150. Berlin: Springer; 2000. pp. 227–250.
-
- Nutt D. J Psychiatr Res. 1990;24:105–110. - PubMed
-
- MacDonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. - PubMed
-
- Costa E, Guidotti A. Trends Pharmacol Sci. 1996;17:192–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

