Activation of Trk neurotrophin receptors in the absence of neurotrophins
- PMID: 11248116
- PMCID: PMC30691
- DOI: 10.1073/pnas.061020198
Activation of Trk neurotrophin receptors in the absence of neurotrophins
Abstract
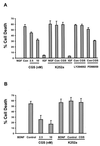
Neurotrophins regulate neuronal cell survival and synaptic plasticity through activation of Trk receptor tyrosine kinases. Binding of neurotrophins to Trk receptors results in receptor autophosphorylation and downstream phosphorylation cascades. Here, we describe an approach to use small molecule agonists to transactivate Trk neurotrophin receptors. Activation of TrkA receptors in PC12 cells and TrkB in hippocampal neurons was observed after treatment with adenosine, a neuromodulator that acts through G protein-coupled receptors. These effects were reproduced by using the adenosine agonist CGS 21680 and were counteracted with the antagonist ZM 241385, indicating that this transactivation event by adenosine involves adenosine 2A receptors. The increase in Trk activity could be inhibited by the use of the Src family-specific inhibitor, PP1, or K252a, an inhibitor of Trk receptors. In contrast to other G protein-coupled receptor transactivation events, adenosine used Trk receptor signaling with a longer time course. Moreover, adenosine activated phosphatidylinositol 3-kinase/Akt through a Trk-dependent mechanism that resulted in increased cell survival after nerve growth factor or brain-derived neurotrophic factor withdrawal. Therefore, adenosine acting through the A(2A) receptors exerts a trophic effect through the engagement of Trk receptors. These results provide an explanation for neuroprotective actions of adenosine through a unique signaling mechanism and raise the possibility that small molecules may be used to elicit neurotrophic effects for the treatment of neurodegenerative diseases.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

