The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs
- PMID: 11248841
- PMCID: PMC29104
- DOI: 10.1186/1471-2431-1-1
The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs
Abstract
Background: Recent reports have raised concerns that postnatal steroids may cause neuro-developmental impairment in preterm infants. This systematic review was performed with the objective of determining whether glucocorticoid therapy, to prevent or treat bronchopulmonary dysplasia, impairs neuro-developmental outcomes in preterm infants.
Method: A systematic review of the literature was performed. Medline was searched and articles retrieved using predefined criteria. Data from randomized controlled trials with adequate neuro-developmental follow up (to at least one year) were entered into a meta-analysis to determine the effects of postnatal treatment of preterm infants with glucocorticoids. Cerebral palsy rates, and neuro-developmental impairment (developmental score more than 2SD below the mean, or cerebral palsy or blindness) were analyzed. The studies were divided into 2 groups according to the extent of contamination of the results by treatment of controls with steroids after the initial study period, those with less than 30% contamination, and those with more than 30% contamination or size of contamination not reported.
Results: Postnatal steroid therapy is associated with an increase in cerebral palsy and neuro-developmental impairment. The studies with less contamination show a greater effect of the steroids, consistent with a real direct toxic effect of steroids on the developing central nervous system. The typical relative risk for the development of cerebral palsy derived from studies with less than 30% contamination is 2.86 (95% CI 1.95, 4.19). The typical relative risk for the development of neuro-developmental disability among followed up infants from studies with less than 30% contamination is 1.66 (95% CI 1.26, 2.19). From this subgroup of studies, the number of premature infants who need to be treated to have one more infant with cerebral palsy (number needed to harm, NNH) is 7; to have one more infant with neuro-developmental impairment the NNH is 11.
Conclusions: Postnatal pharmacologic steroid treatment for prevention or treatment of bronchopulmonary dysplasia is associated with dramatic increases in neuro-developmental impairment. As there is no clear evidence in the literature of long term benefit, their use for this indication should be abandoned.
Figures
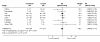
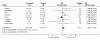
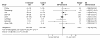
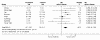

References
-
- Silverman WA, Flynn JT. Overview: a developmental retinopathy reconsidered. In Silverman WA, Flynn JT, Eds, Retinopathy of Prematurity, Blackwell Scientific Publications, Boston, 1985.
-
- Feder HM, Jr, Osier C, Maderazo EG. Chloramphenicol: A review of its use in clinical practice. Rev Infect Dis. 1981;3:479–491. - PubMed
-
- Menon PA, Thach BT, Smith CH, Landt M, Roberts JL, Hillman RE, Hillman LS. Benzyl alcohol toxicity in a neonatal intensive care unit. Incidence, symptomatology, and mortality. Am J Perinatol. 1984;1:288–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

