Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action
- PMID: 11250727
- PMCID: PMC138656
- DOI: 10.1186/bcr79
Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action
Abstract
Natural, synthetic and environmental estrogens have numerous effects on the development and physiology of mammals. Estrogen is primarily known for its role in the development and functioning of the female reproductive system. However, roles for estrogen in male fertility, bone, the circulatory system and immune system have been established by clinical observations regarding sex differences in pathologies, as well as observations following menopause or castration. The primary mechanism of estrogen action is via binding and modulation of activity of the estrogen receptors (ERs), which are ligand-dependent nuclear transcription factors. ERs are found in highest levels in female tissues critical to reproduction, including the ovaries, uterus, cervix, mammary glands and pituitary gland. Since other affected tissues have extremely low levels of ER, indirect effects of estrogen, for example induction of pituitary hormones that affect the bone, have been proposed. The development of transgenic mouse models that lack either estrogen or ER have proven to be valuable tools in defining the mechanisms by which estrogen exerts its effects in various systems. The aim of this article is to review the mouse models with disrupted estrogen signaling and describe the associated phenotypes.
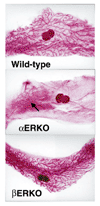
Figures





References
-
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. . Endocrinology. 1996;137:4796–4805. - PubMed
-
- Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor alpha for development or function. . Endocrinology. 2000;141:1273–1276. - PubMed
-
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

