Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin
- PMID: 11250888
- PMCID: PMC145528
- DOI: 10.1093/emboj/20.6.1215
Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin
Abstract
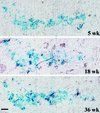
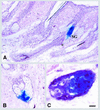
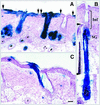
Continuous renewal of the epidermis and its appendages throughout life depends on the proliferation of a distinct population of cells called stem cells. We have used in situ retrovirus-mediated gene transfer to genetically mark cutaneous epithelial stem cells of adolescent mice, and have followed the fate of the marked progeny after at least 37 epidermal turnovers and five cycles of depilation-induced hair growth. Histological examination of serial sections of labeled pilosebaceous units demonstrated a complex cell lineage. In most instances, labeled cells were confined to one or more follicular compartments or solely to sebaceous glands. Labeled keratinocytes in interfollicular epidermis were confined to distinct columnar units representing epidermal proliferative units. The contribution of hair follicles to the epidermis was limited to a small rim of epidermis at the margin of the follicle, indicating that long term maintenance of interfollicular epidermis was independent of follicle-derived cells. Our results indicate the presence of multiple stem cells in cutaneous epithelium, some with restricted lineages in the absence of major injury.
Figures
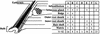


References
-
- Al-Barwari S.E. and Potten,C.S. (1976) Regeneration and dose–response characteristics of irradiated mouse dorsal epidermal cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 30, 201–216. - PubMed
-
- Allen T.D. and Potten,C.S. (1974) Fine-structural identification and organization of the epidermal proliferative unit. J. Cell Sci., 15, 291–319. - PubMed
-
- Burns J.C., Friedmann,T., Driever,W., Burrascano,M. and Yee,J.K. (1993) Vesicular somatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and non-mammalian cells. Proc. Natl Acad. Sci. USA, 90, 8033–8037. - PMC - PubMed
-
- Cepko C.L., Ryder,E., Austin,C., Golden,J., Fields-Berry,S. and Lin,J. (1998). Lineage analysis using retroviral vectors. Methods, 14, 393–406. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

