Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways
- PMID: 11250891
- PMCID: PMC145521
- DOI: 10.1093/emboj/20.6.1245
Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways
Abstract
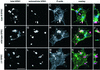
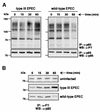
The extracellular pathogen enteropathogenic Escherichia coli (EPEC) uses a type III secretion system to inhibit its uptake by macrophages. We show that EPEC antiphagocytosis is independent of the translocated intimin receptor Tir and occurs by preventing F-actin polymerization required for bacterial uptake. EPEC-macrophage contact triggered activation of phosphatidylinositol (PI) 3-kinase, which was subsequently inhibited in a type III secretion-dependent manner. Inhibition of PI 3-kinase significantly reduced uptake of a secretion-deficient mutant, without affecting antiphagocytosis by the wild type, suggesting that EPEC blocks a PI 3-kinase-dependent phagocytic pathway. EPEC specifically inhibited Fc gamma receptor- but not CR3-receptor mediated phagocytosis of opsonized zymosan. We showed that EPEC inhibits PI 3-kinase activity rather than its recruitment to the site of bacterial contact. Phagocytosis of a secretion mutant correlated with the association of PI 3-kinase with tyrosine-phosphorylated proteins, which wild-type EPEC prevented. These results show that EPEC blocks its uptake by inhibiting a PI 3-kinase-mediated pathway, and translocates effectors other than Tir to interfere with actin-driven host cell processes. This constitutes a novel mechanism of phagocytosis avoidance by an extracellular pathogen.
Figures






References
-
- Caron E. and Hall,A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science, 282, 1717–1721. - PubMed
-
- Celli J., Deng,W. and Finlay,B.B. (2000) Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell. Microbiol., 2, 1–9. - PubMed
-
- Cox D., Tseng,C.C., Bjekic,G. and Greenberg,S. (1999) A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem., 274, 1240–1247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

