5' cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis
- PMID: 11250909
- PMCID: PMC145522
- DOI: 10.1093/emboj/20.6.1439
5' cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis
Abstract
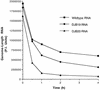
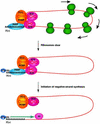
A cloverleaf structure at the 5' terminus of poliovirus RNA binds viral and cellular proteins. To examine the role of the cloverleaf in poliovirus replication, we determined how cloverleaf mutations affected the stability, translation and replication of poliovirus RNA in HeLa S10 translation-replication reactions. Mutations within the cloverleaf destabilized viral RNA in these reactions. Adding a 5' 7-methyl guanosine cap fully restored the stability of the mutant RNAs and had no effect on their translation. These results indicate that the 5' cloverleaf normally protects uncapped poliovirus RNA from rapid degradation by cellular nucleases. Preinitiation RNA replication complexes formed with the capped mutant RNAs were used to measure negative-strand synthesis. Although the mutant RNAs were stable and functional mRNAs, they were not active templates for negative-strand RNA synthesis. Therefore, the 5' cloverleaf is a multifunctional cis-acting replication element required for the initiation of negative-strand RNA synthesis. We propose a replication model in which the 5' and 3' ends of viral RNA interact to form a circular ribonucleoprotein complex that regulates the stability, translation and replication of poliovirus RNA.
Figures









References
-
- Ambros V. and Baltimore,D. (1980) Purification and properties of a HeLa cell enzyme able to remove the 5′-terminal protein from poliovirus RNA. J. Biol. Chem., 255, 6739–6744. - PubMed
-
- Ambros V., Pettersson,R.F. and Baltimore,D. (1978) An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5′ terminal protein. Cell, 15, 1439–1446. - PubMed
-
- Andino R., Rieckhof,G.E. and Baltimore,D. (1990a) A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell, 63, 369–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

