Pharmacodynamics and safety of lefradafiban, an oral platelet glycoprotein IIb/IIIa receptor antagonist, in patients with stable coronary artery disease undergoing elective angioplasty
- PMID: 11250974
- PMCID: PMC1729676
- DOI: 10.1136/heart.85.4.444
Pharmacodynamics and safety of lefradafiban, an oral platelet glycoprotein IIb/IIIa receptor antagonist, in patients with stable coronary artery disease undergoing elective angioplasty
Abstract
Objective: Lefradafiban is the orally active prodrug of fradafiban, a glycoprotein IIb/IIIa receptor antagonist. The present phase II study aimed to determine the dose of lefradafiban that provides 80% blockade of the glycoprotein IIb/IIIa receptors by fradafiban, and to study the pharmacodynamics and safety of different doses in patients with stable angina undergoing angioplasty.
Design: A double blind, placebo controlled, dose finding study.
Setting: Four academic and community hospitals in the Netherlands.
Patients: 64 patients with stable coronary artery disease undergoing elective percutaneous transluminal coronary angioplasty.
Interventions: 30 mg, 45 mg, and 60 mg of lefradafiban three times daily or placebo was given for 48 hours.
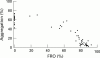
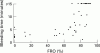
Main outcome measures: The primary safety end point was the occurrence of bleeding, classified as major, minor, or insignificant according to the thrombolysis in myocardial infarction (TIMI) criteria. Efficacy indices included per cent fibrinogen receptor occupancy (FRO), ex vivo platelet aggregation, and plasma concentrations of fradafiban.
Results: Administration of lefradafiban 30, 45, and 60 mg three times daily resulted in a dose dependent increase in median FRO levels of 71%, 85%, and 88%, respectively. Inhibition of platelet aggregation was closely related to FRO. There were no major bleeding events. The 60 mg lefradafiban group had a high (71%) incidence of minor and insignificant bleeding. The incidence of bleeding was 44% in the 30 mg and 45 mg groups, compared with 9% in placebo patients. Puncture site bleeding was the most common event. The odds of bleeding increased by 3% for every 1% increase in FRO.
Conclusions: Lefradafiban is an effective oral glycoprotein IIb/IIIa receptor blocker. The clinical effectiveness of doses up to 45 mg three times daily should be investigated.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
