The facilitated component of intestinal glucose absorption
- PMID: 11251042
- PMCID: PMC2278489
- DOI: 10.1111/j.1469-7793.2001.0585h.x
The facilitated component of intestinal glucose absorption
Abstract
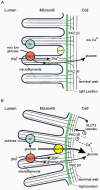
Over the last decade, a debate has developed about the mechanism of the passive or 'diffusive' component of intestinal glucose absorption and, indeed, whether it even exists. Pappenheimer and colleagues have proposed that paracellular solvent drag contributes a passive component, which, at high concentrations of sugars similar to those in the jejunal lumen immediately after a meal, is severalfold greater than the active component mediated by the Na+-glucose cotransporter SGLT1. On the other hand, Ferraris & Diamond maintain that the kinetics of glucose absorption can be explained solely in terms of SGLT1 and that a passive or paracellular component plays little, if any, part. Recently, we have provided new evidence that the passive component of glucose absorption exists, but is in fact facilitated since it is mediated by the rapid, glucose-dependent activation and recruitment of the facilitative glucose transporter GLUT2 to the brush-border membrane; regulation involves a protein kinase C (PKC)-dependent pathway activated by glucose transport through SGLT1 and also involves mitogen-activated protein kinase (MAP kinase) signalling pathways. This topical review seeks to highlight the significant points of the debate, to show how our proposals on GLUT2 impact on different aspects of the debate and to look at the regulatory events that are likely to be involved in the short-term regulation of sugar absorption during the assimilation of a meal.
Figures


References
-
- Brot-Laroche E, Serrano MA, Delhomme B, Alvarado F. Temperature sensitivity and substrate specificity of two distinct Na+-activated d-glucose transport systems in guinea-pig jejunal brush-border membrane vesicles. Journal of Biological Chemistry. 1986;261:6168–6176. - PubMed
-
- Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. American Journal of Physiology. 1997;273:R1965–1971. - PubMed
-
- Corpe CP, Basaleh MM, Affleck J, Gould GW, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflügers Archiv. 1996;432:192–201. - PubMed
-
- Crane RK, Miller D, Bihler I. In: Membrane Transport and Metabolism. Kleinzeller A, Kotyk A, editors. New York: Academic Press; 1961. pp. 439–449.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

