Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans
- PMID: 11251050
- PMCID: PMC2278497
- DOI: 10.1111/j.1469-7793.2001.0677h.x
Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans
Abstract
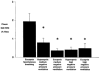
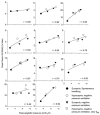
1. Upper airway dilator muscles are phasically activated throughout breathing by respiratory pattern generator neurons. Studies have shown that non-physiological upper airway mechanoreceptive stimuli (e.g. rapidly imposed pulses of negative pressure) also activate these muscles. Such reflexes may become activated during conditions that alter airway resistance in order to stabilise airway patency. 2. To determine the contribution of ongoing mechanoreceptive reflexes to phasic activity of airway dilators, we assessed genioglossal electromyogram (GG EMG: rectified with moving time average of 100 ms) during slow (physiological) oscillations in negative pressure generated spontaneously and passively (negative pressure ventilator). 3. Nineteen healthy adults were studied while awake, during passive mechanical ventilation across normal physiological ranges of breathing rates (13-19 breaths min-1) and volumes (0.5-1.0 l) and during spontaneous breathing across the physiological range of end-tidal carbon dioxide (PET,CO2; 32-45 mmHg). 4. Within-breath phasic changes in airway mechanoreceptor stimuli (negative pressure or flow) were highly correlated with within-breath phasic genioglossal activation, probably representing a robust mechanoreceptive reflex. These reflex relationships were largely unchanged by alterations in central drive to respiratory pump muscles or the rate of mechanical ventilation within the ranges studied. A multivariate model revealed that tonic GG EMG, PET,CO2 and breath duration provided no significant independent information in the prediction of inspiratory peak GG EMG beyond that provided by epiglottic pressure, which alone explained 93 % of the variation in peak GG EMG across all conditions. The overall relationship was: Peak GG EMG = 79.7 - (11.3 X Peak epiglottic pressure), where GG EMG is measured as percentage of baseline, and epiglottic pressure is in cmH2O. 5. These data provide strong evidence that upper airway dilator muscles can be activated throughout inspiration via ongoing mechanoreceptor reflexes. Such a feedback mechanism is likely to be active on a within-breath basis to protect upper airway patency in awake humans. This mechanism could mediate the increased genioglossal activity observed in patients with obstructive sleep apnoea (i.e. reflex compensation for an anatomically smaller airway).
Figures

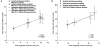


References
-
- Berry RB, McNellis MI, Kouchi K, Light RW. Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. American Journal of Respiratory and Critical Care Medicine. 1997;156:127–132. - PubMed
-
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–31. - PubMed
-
- Curran AK, Eastwood PR, Harms CA, Smith CA, Dempsey JA. Superior laryngeal nerve section alters responses to upper airway distortion in sleeping dogs. Journal of Applied Physiology. 1997;83:768–775. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

