Multifaceted physiological response allows yeast to adapt to the loss of the signal recognition particle-dependent protein-targeting pathway
- PMID: 11251072
- PMCID: PMC30965
- DOI: 10.1091/mbc.12.3.577
Multifaceted physiological response allows yeast to adapt to the loss of the signal recognition particle-dependent protein-targeting pathway
Abstract
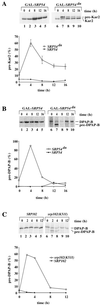
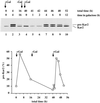
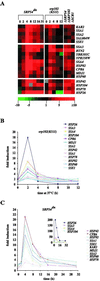
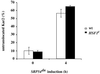
Translational control has recently been recognized as an important facet of adaptive responses to various stress conditions. We describe the adaptation response of the yeast Saccharomyces cerevisiae to the loss of one of two mechanisms to target proteins to the secretory pathway. Using inducible mutants that block the signal recognition particle (SRP) pathway, we find that cells demonstrate a physiological response to the loss of the SRP pathway that includes specific changes in global gene expression. Upon inducing the loss of the SRP pathway, SRP-dependent protein translocation is initially blocked, and cell growth is considerably slowed. Concomitantly, gene expression changes include the induction of heat shock genes and the repression of protein synthesis genes. Remarkably, within hours, the efficiency of protein sorting improves while cell growth remains slow in agreement with the persistent repression of protein synthesis genes. Our results suggest that heat shock gene induction serves to protect cells from mislocalized precursor proteins in the cytosol, whereas reduced protein synthesis helps to regain efficiency in protein sorting by reducing the load on the protein translocation apparatus. Thus, we suggest that cells trade speed in cell growth for fidelity in protein sorting to adjust to life without SRP.
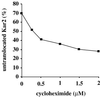
Figures


References
-
- Arnold CE, Wittrup KD. The stress response to loss of signal recognition particle function in Saccharomyces cerevisiae. J Biol Chem. 1994;269:30412–30418. - PubMed
-
- Beckmann R, Bubeck D, Grassucci R, Penczek P, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex [see comments] Science. 1997;278:2123–2126. - PubMed
-
- Bernstein HD, Poritz MA, Strub K, Hoben PJ, Brenner S, Walter P. Model for signal sequence recognition from amino-acid sequence of 54k subunit of signal recognition particle. Nature. 1989;340:482–486. - PubMed
-
- Brodsky JL. Translocation of proteins across the endoplasmic reticulum membrane. Int Rev Cytol. 1998;178:277–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

