Interaction of the E1A oncoprotein with Yak1p, a novel regulator of yeast pseudohyphal differentiation, and related mammalian kinases
- PMID: 11251081
- PMCID: PMC30974
- DOI: 10.1091/mbc.12.3.699
Interaction of the E1A oncoprotein with Yak1p, a novel regulator of yeast pseudohyphal differentiation, and related mammalian kinases
Abstract
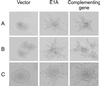
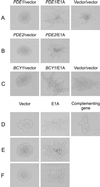
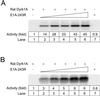
The C-terminal portion of adenovirus E1A suppresses ras-induced metastasis and tumorigenicity in mammalian cells; however, little is known about the mechanisms by which this occurs. In the simple eukaryote Saccharomyces cerevisiae, Ras2p, the homolog of mammalian h-ras, regulates mitogen-activated protein kinase (MAPK) and cyclic AMP-dependent protein kinase A (cAMP/PKA) signaling pathways to control differentiation from the yeast form to the pseudohyphal form. When expressed in yeast, the C-terminal region of E1A induced pseudohyphal differentiation, and this was independent of both the MAPK and cAMP/PKA signaling pathways. Using the yeast two-hybrid system, we identified an interaction between the C-terminal region of E1A and Yak1p, a yeast dual-specificity serine/threonine protein kinase that functions as a negative regulator of growth. E1A also physically interacts with Dyrk1A and Dyrk1B, two mammalian homologs of Yak1p, and stimulates their kinase activity in vitro. We further demonstrate that Yak1p is required in yeast to mediate pseudohyphal differentiation induced by Ras2p-regulated signaling pathways. However, pseudohyphal differentiation induced by the C-terminal region of E1A is largely independent of Yak1p. These data suggest that mammalian Yak1p-related kinases may be targeted by the E1A oncogene to modulate cell growth.
Figures








References
-
- Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1998.
-
- Becker W, Joost H-G. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog Nucleic Acid Res Mol Biol. 1999;62:1–17. - PubMed
-
- Becker W, Weber Y, Wetzel K, Eirmbter K, Tejedor FJ, Joost H-G. Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J Biol Chem. 1998;273:25893–25902. - PubMed
-
- Boyd JM, Subramanian T, Schaeper U, La Regina M, Bayley S, Chinnadurai G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993;12:469–478. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

