Sex determination in the monoecious species cucumber is confined to specific floral whorls
- PMID: 11251091
- PMCID: PMC135508
- DOI: 10.1105/tpc.13.3.481
Sex determination in the monoecious species cucumber is confined to specific floral whorls
Abstract
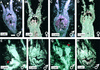
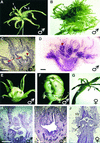
In unisexual flowers, sex is determined by the selective repression of growth or the abortion of either male or female reproductive organs. The mechanism by which this process is controlled in plants is still poorly understood. Because it is known that the identity of reproductive organs in plants is controlled by homeotic genes belonging to the MADS box gene family, we analyzed floral homeotic mutants from cucumber, a species that bears both male and female flowers on the same individual. To study the characteristics of sex determination in more detail, we produced mutants similar to class A and C homeotic mutants from well-characterized hermaphrodite species such as Arabidopsis by ectopically expressing and suppressing the cucumber gene CUCUMBER MADS1 (CUM1). The cucumber mutant green petals (gp) corresponds to the previously characterized B mutants from several species and appeared to be caused by a deletion of 15 amino acid residues in the coding region of the class B MADS box gene CUM26. These homeotic mutants reveal two important concepts that govern sex determination in cucumber. First, the arrest of either male or female organ development is dependent on their positions in the flower and is not associated with their sexual identity. Second, the data presented here strongly suggest that the class C homeotic function is required for the position-dependent arrest of reproductive organs.
Figures





References
-
- Angenent, G.C., Franken, J., Busscher, M., Colombo, L., and van Tunen, A.J. (1993). Petal and stamen formation in petunia is regulated by the homeotic gene fbp1. Plant J. 4, 101–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

