Cell cycle in the fucus zygote parallels a somatic cell cycle but displays a unique translational regulation of cyclin-dependent kinases
- PMID: 11251098
- PMCID: PMC135506
- DOI: 10.1105/tpc.13.3.585
Cell cycle in the fucus zygote parallels a somatic cell cycle but displays a unique translational regulation of cyclin-dependent kinases
Abstract
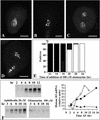
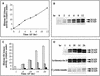
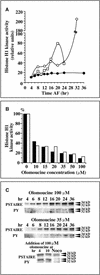
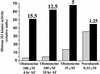
In eukaryotic cells, the basic machinery of cell cycle control is highly conserved. In particular, many cellular events during cell cycle progression are controlled by cyclin-dependent kinases (CDKs). The cell cycle in animal early embryos, however, differs substantially from that of somatic cells or yeasts. For example, cell cycle checkpoints that ensure that the sequence of cell cycle events is correct have been described in somatic cells and yeasts but are largely absent in embryonic cells. Furthermore, the regulation of CDKs is substantially different in the embryonic and somatic cells. In this study, we address the nature of the first cell cycle in the brown alga Fucus, which is evolutionarily distant from the model systems classically used for cell cycle studies in embryos. This cycle consists of well-defined G1, S, G2, and M phases. The purine derivative olomoucine inhibited CDKs activity in vivo and in vitro and induced different cell cycle arrests, including at the G1/S transition, suggesting that, as in somatic cells, CDKs tightly control cell cycle progression. The cell cycle of Fucus zygotes presented the other main features of a somatic cell cycle, such as a functional spindle assembly checkpoint that targets CDKs and the regulation of the early synthesis of two PSTAIRE CDKs, p32 and p34, and the associated histone H1 kinase activity as well as the regulation of CDKs by tyrosine phosphorylation. Surprisingly, the synthesis after fertilization of p32 and p34 was translationally regulated, a regulation not described previously for CDKs. Finally, our results suggest that the activation of mitotic CDKs relies on an autocatalytic amplification mechanism.
Figures


Comment in
-
A brief tour of the cell cycle.Plant Cell. 2001 Mar;13(3):449-51. doi: 10.1105/tpc.13.3.449. Plant Cell. 2001. PMID: 11251087 Free PMC article. No abstract available.
References
-
- Alessi, F., Quarta, S., Savio, M., Riva, F., Rossi, L., Stivala, L.A., Scovassi, A.I., Meijer, L., and Prosperi, E. (1998). The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp. Cell Res. 245, 8–18. - PubMed
-
- Apt, K.E., and Grossman, A.R. (1993). Characterization and transcript analysis of the major phycobiliprotein subunit genes from Aglaothamnion neglectum (Rhodophyta). Plant Mol. Biol. 21, 27–38. - PubMed
-
- Arion, D., and Meijer, L. (1989). M-phase–specific protein kinase from mitotic sea urchin eggs: Cyclic activation depends on protein synthesis and phosphorylation but does not require DNA or RNA synthesis. Exp. Cell Res. 183, 361–375. - PubMed
-
- Arion, D., Meijer, L., Brizuela, L., and Beach, D. (1988). cdc2 is a component of the M phase–specific histone H1 kinase: Evidence for identity with MPF. Cell 55, 371–378. - PubMed
-
- Bell, M.H., Halford, N.G., Ormrod, J.C., and Francis, D. (1993). Tobacco plants transformed with cdc25, a mitotic inducer gene from fission yeast. Plant Mol. Biol. 23, 445–451. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

