The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response
- PMID: 11252722
- PMCID: PMC1083804
- DOI: 10.1093/embo-reports/kve007
The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response
Abstract
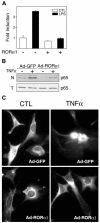
Retinoid-related orphan receptor alpha (ROR alpha) (NR1F1) is a member of the nuclear receptor superfamily whose biological functions are largely unknown. Since staggerer mice, which carry a deletion in the ROR alpha gene, suffer from immune abnormalities, we generated an adenovirus encoding ROR alpha1 to investigate its potential role in control of the inflammatory response. We demonstrated that ROR alpha is expressed in human primary smooth-muscle cells and that ectopic expression of ROR alpha1 inhibits TNFalpha-induced IL-6, IL-8 and COX-2 expression in these cells. ROR alpha1 negatively interferes with the NF-kappaB signalling pathway by reducing p65 translocation as demonstrated by western blotting, immunostaining and electrophoretic mobility shift assays. This action of ROR alpha1 on NF-kappaB is associated with the induction of IkappaB alpha, the major inhibitory protein of the NF-kappaB signalling pathway, whose expression was found to be transcriptionally upregulated by ROR alpha1 via a ROR response element in the IkappaB alpha promoter. Taken together, these data identify ROR alpha1 as a potential target in the treatment of chronic inflammatory diseases, including atherosclerosis and rheumatoid arthritis.
Figures





Similar articles
-
Rev-erbalpha upregulates NF-kappaB-responsive genes in vascular smooth muscle cells.FEBS Lett. 2004 Mar 12;561(1-3):69-74. doi: 10.1016/S0014-5793(04)00118-8. FEBS Lett. 2004. PMID: 15013753
-
Identification of human CYP2C8 as a retinoid-related orphan nuclear receptor target gene.J Pharmacol Exp Ther. 2009 Apr;329(1):192-201. doi: 10.1124/jpet.108.148916. Epub 2009 Jan 22. J Pharmacol Exp Ther. 2009. PMID: 19164466 Free PMC article.
-
Identification of Reverb(alpha) as a novel ROR(alpha) target gene.J Biol Chem. 2002 Sep 20;277(38):35013-8. doi: 10.1074/jbc.M202979200. Epub 2002 Jul 11. J Biol Chem. 2002. PMID: 12114512
-
The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes.Prog Nucleic Acid Res Mol Biol. 2001;69:205-47. doi: 10.1016/s0079-6603(01)69048-2. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11550795 Review.
-
Recent advances in the mechanisms of action and physiological functions of the retinoid-related orphan receptors (RORs).Curr Drug Targets Inflamm Allergy. 2004 Dec;3(4):395-412. doi: 10.2174/1568010042634497. Curr Drug Targets Inflamm Allergy. 2004. PMID: 15584888 Review.
Cited by
-
Modeling the circadian regulation of the immune system: Sexually dimorphic effects of shift work.PLoS Comput Biol. 2021 Mar 31;17(3):e1008514. doi: 10.1371/journal.pcbi.1008514. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33788832 Free PMC article.
-
From Chronodisruption to Sarcopenia: The Therapeutic Potential of Melatonin.Biomolecules. 2023 Dec 12;13(12):1779. doi: 10.3390/biom13121779. Biomolecules. 2023. PMID: 38136651 Free PMC article. Review.
-
Mucosal Genes Encoding Clock, Inflammation and Their Mutual Regulators Are Disrupted in Pediatric Patients with Active Ulcerative Colitis.Int J Mol Sci. 2024 Jan 25;25(3):1488. doi: 10.3390/ijms25031488. Int J Mol Sci. 2024. PMID: 38338765 Free PMC article.
-
Chronobiology of limbic seizures: Potential mechanisms and prospects of chronotherapy for mesial temporal lobe epilepsy.Neurosci Biobehav Rev. 2019 Mar;98:122-134. doi: 10.1016/j.neubiorev.2019.01.004. Epub 2019 Jan 7. Neurosci Biobehav Rev. 2019. PMID: 30629979 Free PMC article. Review.
-
Retinoic acid receptor-related orphan receptor α reduces lipid droplets by upregulating neutral cholesterol ester hydrolase 1 in macrophages.BMC Mol Cell Biol. 2020 Apr 22;21(1):32. doi: 10.1186/s12860-020-00276-z. BMC Mol Cell Biol. 2020. PMID: 32321446 Free PMC article.
References
-
- Becker-André M., Wiesenberg, I., Schaeren-Wiemers, N. André, E., Missbach, M., Saurat, J.-H. and Carlberg, C. (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem., 269, 28531–28534. - PubMed
-
- Becker-André M., Wiesenberg, I., Schaeren-Wiemers, N. André, E., Missbach, M., Saurat, J.-H. and Carlberg, C. (1997) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily (additions and corrections). J. Biol. Chem., 272, 16707. - PubMed
-
- Carlberg C., Van Huijsduijnen, R.H., Staple, J.K., De Lamarter, J.F. and Becker-André, M. (1994) RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol. Endocrinol., 8, 757–770. - PubMed
-
- Chinetti G. et al. (1998) Activation of peroxisome proliferator activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem., 273, 25573–25580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

