The establishment of Spemann's organizer and patterning of the vertebrate embryo
- PMID: 11252746
- PMCID: PMC2291143
- DOI: 10.1038/35042039
The establishment of Spemann's organizer and patterning of the vertebrate embryo
Abstract
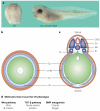
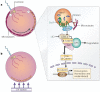
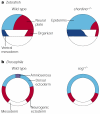
Molecular studies have begun to unravel the sequential cell-cell signalling events that establish the dorsal-ventral, or 'back-to-belly', axis of vertebrate animals. In Xenopus and zebrafish, these events start with the movement of membrane vesicles associated with dorsal determinants. This mediates the induction of mesoderm by generating gradients of growth factors. Dorsal mesoderm then becomes a signalling centre, the Spemann's organizer, which secretes several antagonists of growth-factor signalling. Recent studies have led to new models for the regulation of cell-cell signalling during development, which may also apply to the homeostasis of adult tissues.
Figures


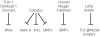

References
-
- Spemann H. Vererbung und Entwicklungsmechanik. Naturwissenchaften. 1924;12:65–79.
-
- Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford Univ. Press; Oxford: 1988. A magnificent account of the experiments that have shaped embryological thinking in the twentieth century.
-
- Brachet J. An old enigma: the gray crescent of amphibian eggs. Curr. Top. Dev. Biol. 1977;11:133–186. - PubMed
-
- Gerhart J, Doniach T, Stewart R. In: Gastrulation: Movements, Patterns, and Molecules. Keller R, Clark WH, Griffin F, editors. Plenum; New York: 1991. pp. 57–76.
-
- Black SD, Gerhart J. High frequency twinning of Xenopus laevis embryos from eggs centrifuged before first cleavage. Dev. Biol. 1986;116:228–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

