Differential regulation of Bvg-activated virulence factors plays a role in Bordetella pertussis pathogenicity
- PMID: 11254549
- PMCID: PMC98121
- DOI: 10.1128/IAI.69.4.1983-1993.2001
Differential regulation of Bvg-activated virulence factors plays a role in Bordetella pertussis pathogenicity
Abstract
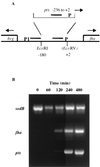
Bordetella pertussis, the causative agent of whooping cough, regulates expression of many virulence factors via a two-component signal transduction system encoded by the bvgAS regulatory locus. It has been shown by transcription activation kinetics that several of the virulence factors are differentially regulated. fha is transcribed within 10 min following a bvgAS-inducing signal, while prn is transcribed after 1 h and ptx is not transcribed until 2 to 4 h after induction. These genes therefore represent early, intermediate, and late classes of bvg-activated promoters, respectively. Although there have been many insightful studies into the mechanisms of BvgAS-mediated regulation, the role that differential regulation of virulence genes plays in B. pertussis pathogenicity has not been characterized. We provide evidence that alterations to the promoter regions of bvg-activated genes can alter the kinetic pattern of expression of these genes without changing steady-state transcription levels. In addition, B. pertussis strains containing these promoter alterations that express either ptx at an early time or fha at a late time demonstrate a significant reduction in their ability to colonize respiratory tracts in an intranasal mouse model of infection. These data suggest a role for differential regulation of bvg-activated genes, and therefore for the BvgAS regulatory system, in the pathogenicity of B. pertussis.
Figures



References
-
- Aintablian N, Walpita P, Sawyer M H. Detection of Bordetella pertussis and respiratory syncytial virus in air samples from hospital rooms. Infect Control Hosp Epidemiol. 1998;19:918–923. - PubMed
-
- Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. - PubMed
-
- Bartoloni A, Pizza M, Bigio M, Nucci D, Ashworth L A, Irons L I, Robinson A, Burns D, Manclark C, Sato H, Rappuoli R. Binding of a protective epitope of pertussis toxin by in vitro refolding of recombinant fragments. Bio/Technology. 1988;6:709–712.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

