Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways
- PMID: 11254551
- PMCID: PMC98123
- DOI: 10.1128/IAI.69.4.2001-2010.2001
Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways
Abstract
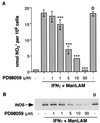
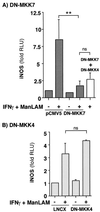
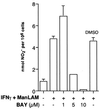
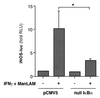
Nitric oxide (NO*) expression by inducible nitric oxide synthase (iNOS) is an important host defense mechanism against Mycobacterium tuberculosis in mononuclear phagocytes. The objective of this investigation was to examine the role of mitogen-activated protein (MAP) kinase (MAPK) and nuclear factor kappaB (NF-kappaB) signaling pathways in the regulation of iNOS and NO* by a mycobacterial cell wall lipoglycan known as mannose-capped lipoarabinomannan (ManLAM). Specific pharmacologic inhibition of the extracellular-signal-regulated kinase (ERK) or NF-kappaB pathway revealed that both these signaling cascades were required in gamma interferon (IFN-gamma)-ManLAM-induced iNOS protein and NO2- expression in mouse macrophages. Transient cotransfection of dominant-negative protein mutants of the c-Jun NH2-terminal kinase (JNK) pathway revealed that the MAP kinase kinase 7 (MKK7)-JNK cascade also mediated IFN-gamma-ManLAM induction of iNOS promoter activity whereas MKK4 did not. Overexpression of null mutant IkappaBalpha, a potent inhibitor of NF-kappaB activation, confirmed that the IkappaBalpha kinase (IKK)-NF-kappaB signaling pathway enhanced IFN-gamma-ManLAM-induced iNOS promoter activity. By contrast, activated p38mapk inhibited iNOS induction. These results indicate that combined IFN-gamma and ManLAM stimulation induced iNOS and NO. expression and that MEK1-ERK, MKK7-JNK, IKK-NF-kappaB, and p38mapk signaling pathways play important regulatory roles.
Figures




References
-
- Adams L B, Dinauer M C, Morgenstern D E, Krahenbuhl J L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuberc Lung Dis. 1997;78:237–246. - PubMed
-
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Alpert D, Schwenger P, Han J, Vilcek J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IκB phosphorylation and NF-κB activation. J Biol Chem. 1999;274:22176–22183. - PubMed
-
- Arias M, Rojas M, Zabaleta J, Rodriguez J I, Paris S C, Barrera L F, Garcia L F. Inhibition of virulent Mycobacterium tuberculosis by Bcg (r) and Bcg (s) macrophages correlates with nitric oxide production. J Infect Dis. 1997;176:1552–1558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

