Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus
- PMID: 11254556
- PMCID: PMC98128
- DOI: 10.1128/IAI.69.4.2037-2044.2001
Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus
Abstract
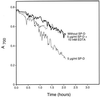
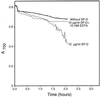
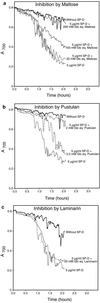
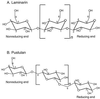
Surfactant proteins A (SP-A) and D (SP-D) are members of the collectin family of calcium-dependent lectins and are important pulmonary host defense molecules. Human SP-A and SP-D and rat SP-D bind to Aspergillus fumigatus conidia, but the ligand remains unidentified. To identify a fungal ligand for SP-A and/or SP-D, we examined the interactions of the proteins with Saccharomyces cerevisiae. SP-D but not SP-A bound yeast cells, and EDTA inhibited the binding. SP-D also aggregated yeast cells and isolated yeast cell walls. Treating yeast cells to remove cell wall mannoprotein did not reduce SP-D binding, and SP-D failed to aggregate chitin. However, SP-D aggregated yeast glucan before and after treatment with a beta(1-->3)-glucanase, suggesting a specific interaction between the collectin and beta(1-->6)-glucan. In support of this idea, SP-D-induced yeast aggregation was strongly inhibited by pustulan [a beta(1-->6)-linked glucose homopolymer] but was not inhibited by laminarin [a beta(1-->3)-linked glucose homopolymer]. Additionally, pustulan but not laminarin strongly inhibited SP-D binding to A. fumigatus. The pustulan concentration for 50% inhibition of SP-D binding to A. fumigatus is 1.0 +/- 0.3 microM glucose equivalents. Finally, SP-D showed reduced binding to the beta(1-->6)-glucan-deficient kre6 yeast mutant. Taken together, these observations demonstrate that beta(1-->6)-glucan is an important fungal ligand for SP-D and that glycosidic bond patterns alone can determine if an extended carbohydrate polymer is recognized by SP-D.
Figures





References
-
- Cabib E, Roberts R, Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. - PubMed
-
- Cambell I, Duffus J H. Yeast. Oxford, United Kingdom: IRL Press Limited; 1988.
-
- Crouch E C. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol. 1998;19:177–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

