Epitope mapping of monoclonal antibodies capable of neutralizing cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli
- PMID: 11254559
- PMCID: PMC98131
- DOI: 10.1128/IAI.69.4.2066-2074.2001
Epitope mapping of monoclonal antibodies capable of neutralizing cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli
Abstract
Cytotoxic necrotizing factor type 1 (CNF1) of uropathogenic Escherichia coli belongs to a family of bacterial toxins that target the small GTP-binding Rho proteins that regulate the actin cytoskeleton. Members of this toxin family typically inactivate Rho; however, CNF1 and the highly related CNF2 activate Rho by deamidation. Other investigators have reported that the first 190 amino acids of CNF1 constitute the cellular binding domain and that the CNF1 enzymatic domain lies within a 300-amino-acid stretch in the C terminus of the toxin. Amino acids 53 to 75 appear to be critical for cell receptor recognition, while amino acids Cys866 and His881 are considered essential for deamidation activity. To delineate further the functional domains of CNF1, we generated 16 monoclonal antibodies (MAbs) against the toxin and used them for epitope mapping studies. Based on Western blot immunoreactivity patterns obtained from a series of truncated CNF1 proteins, this panel of MAbs mapped to epitopes located throughout the toxin, including the binding and enzymatic domains. All MAbs showed reactivity to CNF1 by Western and dot blot analyses. However, only 7 of the 16 MAbs exhibited cross-reactivity with CNF2. Furthermore, only three MAbs demonstrated the capacity to neutralize toxin in either HEp-2 cell assays (inhibition of multinucleation) or 5637 bladder cell assays (inhibition of cytotoxicity). Since CNF1 epitopes recognized by neutralizing MAbs are likely to represent domains or regions necessary for the biological activities of the toxin, the epitopes recognized by these three MAbs, designated JC4 (immunoglobulin G2a [IgG2a]), BF8 (IgA), and NG8 (IgG2a), were more precisely defined. MAbs JC4 and BF8 reacted with epitopes that were common to CNF1 and CNF2 and located within the putative CNF1 binding domain. MAb JC4 recognized an epitope spanning amino acids 169 to 191, whereas MAb BF8 mapped to an epitope between amino acids 135 and 164. Despite the capacity of both MAbs to recognize CNF2 in Western blot analyses, only MAb BF8 neutralized CNF2. MAb NG8 showed reactivity to a CNF1-specific epitope located between amino acids 683 and 730, a region that includes a very small portion of the putative enzymatic domain. Taken together, these findings identify three new regions of the toxin that appear to be critical for the biological activity of CNF1.
Figures

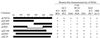


References
-
- Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. - PubMed
-
- Alonso P, Blanco J, Blanco M, Gonzalez E A. Frequent production of toxins by Escherichia coli strains isolated from human urinary tract infections: relation with haemagglutination. FEMS Microbiol Lett. 1987;48:391–396.
-
- Andreu A, Stapleton A E, Fennell C, Lockman H A, Xercavins M, Fernandez F, Stamm W E. Urovirulence determinants in Escherichia coli strains causing prostatitis. J Infect Dis. 1997;176:464–469. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1989.
-
- Blanco J, Blanco M, González E A, Pilar Alonso M, Garabal J I. Comparative evaluation of three tests for the detection of Escherichia coli cytotoxic necrotizing factors (CNF1 and CNF2) using filtrates of cultures treated with mitomycin C. FEMS Microbiol Lett. 1990;69:311–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

