Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin
- PMID: 11254631
- PMCID: PMC98203
- DOI: 10.1128/IAI.69.4.2650-2658.2001
Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin
Abstract
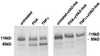
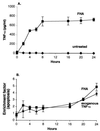
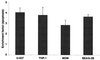
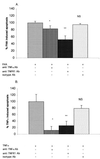
Filamentous hemagglutinin (FHA) is a dominant cell surface-associated Bordetella pertussis adhesin. Recognition that this protein is secreted in significant amounts and that bacterial adhesins may have other activities, prompted an assessment of FHA effects on human macrophages. Incubation of human macrophage-like U937 cells with preparations of FHA resulted in dose-dependent cytotoxicity, with death of 95% of treated cells after 24 h. Based on the use of four independent methods, death of these cells could be largely attributed to apoptosis. FHA-associated apoptosis was also observed in THP-1 macrophage-like cells, fresh human peripheral blood monocyte-derived macrophages (MDM), and BEAS-2B human bronchial epithelial cells. Infection of MDM with wild-type B. pertussis resulted in apoptosis within 6 h, while infection with an FHA-deficient derivative strain was only 50% as effective. FHA-associated cytotoxicity was preceded by host cell secretion of tumor necrosis factor alpha (TNF-alpha), a potential proapoptotic factor. However, pretreatment of cells with a neutralizing anti-TNF-alpha monoclonal antibody inhibited only 16% of the FHA-associated apoptosis. On the other hand, a blocking monoclonal antibody directed against TNF-alpha receptor 1 inhibited FHA-associated apoptosis by 47.7% (P = 0.0001), suggesting that this receptor may play a role in the death pathway activated by FHA. Our in vitro data indicate that secreted and cell-associated FHA elicits proinflammatory and proapoptotic responses in human monocyte-like cells, MDM, and bronchial epithelial cells and suggest a previously unrecognized role for this prominent virulence factor in the B. pertussis-host interaction.
Figures


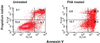



References
-
- Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. - PubMed
-
- Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. - PubMed
-
- Flak T A, Goldman W E. Signaling and cellular specificity of airway nitric oxide production in pertussis. Cell Microbiol. 1999;1:51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

