Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease
- PMID: 11254669
- PMCID: PMC208944
- DOI: 10.1172/JCI10956
Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease
Abstract
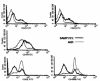
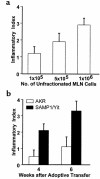
We describe here the immunologic characterization of a new mouse strain, SAMP1/Yit, which spontaneously develops a chronic intestinal inflammation localized to the terminal ileum. The resulting ileitis bears a remarkable resemblance to human Crohn's disease. This strain of mice develops discontinuous, transmural inflammatory lesions in the terminal ileum with 100% penetrance by 30 weeks of age. The intestinal inflammation is characterized by massive infiltration of activated CD4+ and CD8alpha(+)TCRalphabeta(+) T cells into the lamina propria and is accompanied by a dramatic decrease in the intraepithelial lymphocyte CD8alpha(+)TCRgammadelta(+)/CD8alpha(+)TCRalphabeta(+) ratio. The results of adoptive transfer experiments strongly suggest that CD4+ T cells that produce a Th1-like profile of cytokines, e.g., IFN-gamma and TNF, mediate the intestinal inflammation found in SAMP1/Yit mice. In addition, pretreatment of adoptive transfer recipients with a neutralizing anti-TNF antibody prevents the development of intestinal inflammation, suggesting that TNF plays an important role in the pathogenesis of intestinal inflammation in this model. To our knowledge, these data provide the first direct evidence that Th1-producing T cells mediate intestinal inflammation in a spontaneous animal model of human Crohn's disease.
Figures


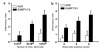

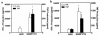

Comment in
-
The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease.J Clin Invest. 2001 Mar;107(6):667-70. doi: 10.1172/JCI12559. J Clin Invest. 2001. PMID: 11254665 Free PMC article. Review. No abstract available.
Similar articles
-
Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation.Gastroenterology. 2005 Mar;128(3):654-66. doi: 10.1053/j.gastro.2004.11.053. Gastroenterology. 2005. PMID: 15765401
-
Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's-like murine ileitis.J Leukoc Biol. 2015 Jun;97(6):1011-22. doi: 10.1189/jlb.3HI0614-303R. Epub 2015 Jan 30. J Leukoc Biol. 2015. PMID: 25637591 Free PMC article.
-
Down-regulation of intestinal lymphocyte activation and Th1 cytokine production by antibiotic therapy in a murine model of Crohn's disease.J Immunol. 2002 Nov 1;169(9):5308-14. doi: 10.4049/jimmunol.169.9.5308. J Immunol. 2002. PMID: 12391251
-
Pathogenesis of gastritis in ileitis-prone SAMP1/Yit mice.Keio J Med. 2011;60(2):65-8. doi: 10.2302/kjm.60.65. Keio J Med. 2011. PMID: 21720202 Review.
-
SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis.Inflamm Bowel Dis. 2011 Dec;17(12):2566-84. doi: 10.1002/ibd.21638. Epub 2011 May 6. Inflamm Bowel Dis. 2011. PMID: 21557393 Free PMC article. Review.
Cited by
-
Intestinal Stem Cell Niche Defects Result in Impaired 3D Organoid Formation in Mouse Models of Crohn's Disease-like Ileitis.Stem Cell Reports. 2020 Aug 11;15(2):389-407. doi: 10.1016/j.stemcr.2020.06.017. Epub 2020 Jul 16. Stem Cell Reports. 2020. PMID: 32679063 Free PMC article.
-
TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis.Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8366-71. doi: 10.1073/pnas.1432897100. Epub 2003 Jun 27. Proc Natl Acad Sci U S A. 2003. PMID: 12832622 Free PMC article.
-
Averting inflammation by targeting the cytokine environment.Nat Rev Drug Discov. 2010 Sep;9(9):703-18. doi: 10.1038/nrd2805. Nat Rev Drug Discov. 2010. PMID: 20811382 Review.
-
SHIP deficiency causes Crohn's disease-like ileitis.Gut. 2011 Feb;60(2):177-88. doi: 10.1136/gut.2009.202283. Epub 2010 Oct 12. Gut. 2011. PMID: 20940287 Free PMC article.
-
Role of interleukin 6 in a murine model of Crohn's ileitis: are cytokine/anticytokine strategies the future for IBD therapies?Gut. 2006 Sep;55(9):1226-7. doi: 10.1136/gut.2005.083121. Gut. 2006. PMID: 16905692 Free PMC article.
References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:928–937. - PubMed
-
- Satsangi J, Jewell D, Parkes M, Bell J. Genetics of inflammatory bowel disease. A personal view on progress and prospects. Dig Dis. 1998;16:370–374. - PubMed
-
- Satsangi J, et al. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet. 1996;347:1212–1217. - PubMed
-
- Duerr RH. Genetics of inflammatory bowel disease. Inflamm Bowel Dis. 1996;2:48–60. - PubMed
-
- Papadakis KA, Targan SR. Current theories on the cause of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:283–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

