Clonality and altered behavior of endothelial cells from hemangiomas
- PMID: 11254674
- PMCID: PMC208946
- DOI: 10.1172/JCI11432
Clonality and altered behavior of endothelial cells from hemangiomas
Abstract
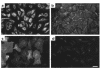
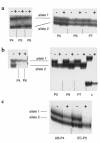
Hemangioma, the most common tumor of infancy, is a benign vascular neoplasm of unknown etiology. We show, for the first time to our knowledge, that endothelial cells from proliferating hemangioma are clonal, and we demonstrate that these hemangioma-derived cells differ from normal endothelial cells in their rates of proliferation and migration in vitro. Furthermore, migration of hemangioma endothelial cells is stimulated by the angiogenesis inhibitor endostatin, unlike the inhibition seen with normal endothelial cells. We conclude that hemangiomas constitute clonal expansions of endothelial cells. This is consistent with the possibility that these tumors are caused by somatic mutations in one or more genes regulating endothelial cell proliferation.
Figures
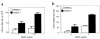

Comment in
-
Pathogenesis of hemangioma.J Clin Invest. 2001 Mar;107(6):665-6. doi: 10.1172/JCI12470. J Clin Invest. 2001. PMID: 11254664 Free PMC article. Review. No abstract available.
References
-
- Mulliken, J.B. 1988. Diagnosis and natural history of hemangiomas. In Vascular birthmarks: hemangiomas and malformations. J.B. Mulliken and A.E. Young, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 41–62.
-
- Razon MJ, Kräling BM, Mulliken JB, Bischoff J. Increased apoptosis coincides with onset of involution in infantile hemangioma. Microcirculation. 1998;5:189–195. - PubMed
-
- Mulliken, J.B., Fishman, S.J., and Burrows, P.E. 2000. Vascular anomalies. In Current problems in surgery. S.A. Wells and L.L. Creswell, editors. Mosby. St. Louis, Missouri, USA. 517–584. - PubMed
-
- Kräling BM, Bischoff J. A simplified method for growth of human microvascular endothelial cells results in decreased senescence and continued responsiveness to cytokines and growth factors. In Vitro Cell Dev Biol Anim. 1998;34:308–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

