An alternative pathway for preclinical research in fluid management
- PMID: 11255593
- PMCID: PMC3226175
- DOI: 10.1186/cc970
An alternative pathway for preclinical research in fluid management
Abstract
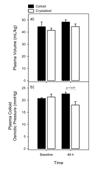
Recent meta-analyses have created uncertainties regarding the appropriate clinical role of colloid resuscitation fluids in critically ill patients and prompted changes in fluid management practice. Such changes may not be justified in view of methodological limitations inherent in the meta-analyses. Further research is nevertheless needed to resolve the questions raised concerning the relationship between choice of resuscitation fluid and patient outcome. Animal studies can play an important part by reliably indicating whether particular fluids are likely to prove effective and safe in clinical trials. It is important to avoid costly large-scale clinical trials that fail to demonstrate the clinical utility of the tested therapy, as resources expended in failed trials raise overall development costs and thereby restrict the range of therapies meeting criteria of commercial feasibility. Promising therapies may thus not be pursued, even though an urgent clinical need may exist. An alternative pathway of preclinical research may be of value in avoiding some of the major clinical trial failures of recent years, particularly in the area of sepsis. This alternative pathway commences with the formulation of hypotheses by therapeutics developers. Independent preclinical investigators are challenged, by means of a competitive request for proposals, to test the hypotheses in rigorous randomized studies employing clinically relevant animal models. Promising proposals would then be selected for further development with the aid of peer review. The results of the randomized animal studies, along with other preclinical data, could also be evaluated using accepted principles of 'critical appraisal' commonly applied to clinical trial results. This critical appraisal might, where appropriate, include meta-analysis of animal study findings. This alternative preclinical pathway to new product evaluation should be completed before the commencement of large-scale clinical trials.
Figures
References
-
- Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

