A wheel invented three times. The molecular structures of the three carbonic anhydrases
- PMID: 11256616
- PMCID: PMC1083691
- DOI: 10.1093/embo-reports/kvd016
A wheel invented three times. The molecular structures of the three carbonic anhydrases
Figures
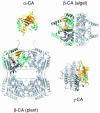

References
-
- Hewett-Emmett D. and Tashian, R.E. (1996) Functional diversity, conservation, and convergence in the evolution of the α-, β-, and γ-carbonic anhydrase gene families. Mol. Phylogenet. Evol., 5, 50–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

