Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir
- PMID: 11257030
- PMCID: PMC90439
- DOI: 10.1128/AAC.45.4.1162-1167.2001
Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir
Abstract
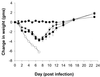
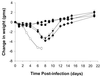
We have recently reported an influenza virus neuraminidase inhibitor, RWJ-270201 (BCX-1812), a novel cyclopentane derivative discovered through structure-based drug design. In this paper, we compare the potency of three compounds, RWJ-270201, oseltamivir, and zanamivir, against neuraminidase enzymes from various subtypes of influenza. RWJ-270201 effectively inhibited all tested influenza A and influenza B neuraminidases in vitro, with 50% inhibitory concentrations of 0.09 to 1.4 nM for influenza A neuraminidases and 0.6 to 11 nM for influenza B neuraminidases. These values were comparable to or lower than those for oseltamivir carboxylate (GS4071) and zanamivir (GG167). RWJ-270201 demonstrated excellent selectivity (>10,000-fold) for influenza virus neuraminidase over mammalian, bacterial, or other viral neuraminidases. Oral administration of a dosage of 1 mg/kg of body weight/day of RWJ-270201 for 5 days (beginning 4 h preinfection) showed efficacy in the murine model of influenza virus infection as determined by lethality and weight loss protection. RWJ-270201 administered intranasally at 0.01 mg/kg/day in the murine influenza model demonstrated complete protection against lethality, whereas oseltamivir carboxylate and zanamivir at the same dose demonstrated only partial protection. In the delayed-treatment murine influenza model, oral administration of a 10-mg/kg/day dose of RWJ-270201 or oseltamivir (GS4104, a prodrug of GS4071) at 24 h postinfection showed significant protection against lethality (P < 0.001 versus control). However, when the treatment was delayed for 48 h, no significant protection was observed in either drug group. No drug-related toxicity was observed in mice receiving 100 mg/kg/day of RWJ-270201 for 5 days. These efficacy and safety profiles justify further consideration of RWJ-270201 for the treatment and prevention of human influenza.
Figures
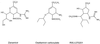
References
-
- Babu Y S, Chand P, Bantia S, Kotian P L, Dehghani A, El-Kattan Y, Lin T, Hutchinson T L, Elliot A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. - PubMed
-
- Hayden F G, Atmar R L, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills R G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. - PubMed
-
- Hayden F G, Treanor J J, Betts R F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. - PubMed
-
- Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. - PubMed
-
- Johansson B E, Grajower B, Kilbourne E D. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine. 1993;11:1037–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

