A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1
- PMID: 11257118
- PMCID: PMC2199199
- DOI: 10.1083/jcb.152.6.1169
A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1
Abstract
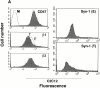
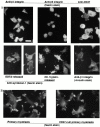
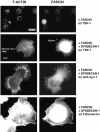
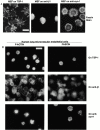
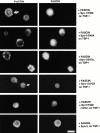
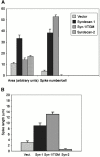
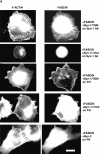
An important role of cell matrix adhesion receptors is to mediate transmembrane coupling between extracellular matrix attachment, actin reorganization, and cell spreading. Thrombospondin (TSP)-1 is a modulatory component of matrix expressed during development, immune response, or wound repair. Cell adhesion to TSP-1 involves formation of biochemically distinct matrix contacts based on stable fascin spikes. The cell surface adhesion receptors required have not been identified. We report here that antibody clustering of syndecan-1 proteoglycan specifically transduces organization of cortical actin and fascin bundles in several cell types. Transfection of COS-7 cells with syndecan-1 is sufficient to stimulate cell spreading, fascin spike assembly, and extensive protrusive lateral ruffling on TSP-1 or on syndecan-1 antibody. The underlying molecular mechanism depends on glycosaminoglycan (GAG) modification of the syndecan-1 core protein at residues S45 or S47 for cell membrane spreading and on the VC2 region of the cytoplasmic domain for spreading and fascin spike formation. Expression of the VC2 deletion mutant or GAG-negative syndecan-1 showed that syndecan-1 is necessary in spreading and fascin spike formation by C2C12 cells on TSP-1. These results establish a novel role for syndecan-1 protein in coupling a physiological matrix ligand to formation of a specific matrix contact structure.
Figures





References
-
- Adams J.C. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 1995;108:1977–1990. - PubMed
-
- Adams J.C. Thrombospondin-1 Int. J. Biochem. Cell Biol 29 1997. 861 865a - PubMed
-
- Adams J.C., Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J. Cell Sci. 1993;104:1061–1071. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

