The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance
- PMID: 11257122
- PMCID: PMC2199198
- DOI: 10.1083/jcb.152.6.1219
The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance
Abstract
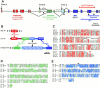
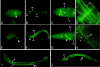
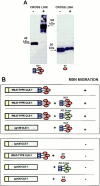
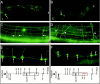
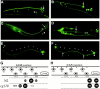
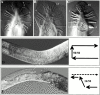
Type XVIII collagen is a homotrimeric basement membrane molecule of unknown function, whose COOH-terminal NC1 domain contains endostatin (ES), a potent antiangiogenic agent. The Caenorhabditis elegans collagen XVIII homologue, cle-1, encodes three developmentally regulated protein isoforms expressed predominantly in neurons. The CLE-1 protein is found in low amounts in all basement membranes but accumulates at high levels in the nervous system. Deletion of the cle-1 NC1 domain results in viable fertile animals that display multiple cell migration and axon guidance defects. Particular defects can be rescued by ectopic expression of the NC1 domain, which is shown to be capable of forming trimers. In contrast, expression of monomeric ES does not rescue but dominantly causes cell and axon migration defects that phenocopy the NC1 deletion, suggesting that ES inhibits the promigratory activity of the NC1 domain. These results indicate that the cle-1 NC1/ES domain regulates cell and axon migrations in C. elegans.
Figures


References
-
- Barstead R.J. Reverse genetics. In: Hope I.A., editor. C. elegansA Practical Approach. Oxford University Press; Oxford, UK: 1999. pp. 97–118.
-
- Baum P.D., Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. - PubMed
-
- Bettinger J.C., Lee K., Rougvie A.E. Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development. 1996;122:2517–2527. - PubMed
-
- Boehm T., Folkman J., Browder T., O'Reilly M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

