Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy
- PMID: 11257139
- PMCID: PMC2193412
- DOI: 10.1084/jem.193.6.727
Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy
Abstract
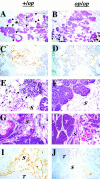
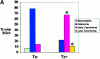
In human breast carcinomas, overexpression of the macrophage colony-stimulating factor (CSF-1) and its receptor (CSF-1R) correlates with poor prognosis. To establish if there is a causal relationship between CSF-1 and breast cancer progression, we crossed a transgenic mouse susceptible to mammary cancer with mice containing a recessive null mutation in the CSF-1 gene (Csf1(op)) and followed tumor progression in wild-type and null mutant mice. The absence of CSF-1 affects neither the incidence nor the growth of the primary tumors but delayed their development to invasive, metastatic carcinomas. Transgenic expression of CSF-1 in the mammary epithelium of both Csf1(op)/Csf1(op) and wild-type tumor-prone mice led to an acceleration to the late stages of carcinoma and to a significant increase in pulmonary metastasis. This was associated with an enhanced infiltration of macrophages into the primary tumor. These studies demonstrate that the growth of mammary tumors and the development to malignancy are separate processes and that CSF-1 selectively promotes the latter process. CSF-1 may promote metastatic potential by regulating the infiltration and function of tumor-associated macrophages as, at the tumor site, CSF-1R expression was restricted to macrophages. Our data suggest that agents directed at CSF-1/CSF-1R activity could have important therapeutic effects.
Figures








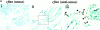
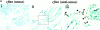






Comment in
-
Inflammatory cells and cancer: think different!J Exp Med. 2001 Mar 19;193(6):F23-6. doi: 10.1084/jem.193.6.f23. J Exp Med. 2001. PMID: 11257144 Free PMC article. Review. No abstract available.
References
-
- Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer statistics, 2000. CA Cancer J. Clin. 2000;50:7–33. - PubMed
-
- Xie B., Tsao S.W., Wong Y.C. Sex hormone-induced mammary carcinogenesis in female Noble ratsexpression of TGF-beta1 and its receptors, TGF-alpha, and EGF-R in mammary carcinogenesis. Breast Cancer Res. Treat. 1999;58:227–239. - PubMed
-
- Dickson C., Creer A., Fantl V. Mammary gland oncogenes as indicators of pathways important in mammary gland development. Oncogene. 2000;19:1097–1101. - PubMed
-
- Hovey R.C., Davey H.W., Mackenszie D.D.S., McFadden T.B. Ontogeny and epithelial-stromal interactions regulate IGF expression in the ovine mammary gland. Mol. Cell. Endocrinol. 1998;136:139–144. - PubMed
-
- Stanley E.R., Guilbert L.T., Tushinski R.J., Bartelmez S.H. CSF-1 A mononuclear phagocyte lineage-specific hemopoietic growth factor. J. Cell. Biochem. 1983;21:151–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

