The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters
- PMID: 11258478
- PMCID: PMC1083770
- DOI: 10.1093/embo-reports/kvd098
The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters
Abstract
Since its discovery more than 10 years ago, the atypical PKC (aPKC) subfamily has attracted great interest. A number of reports have shown that the kinases of this subfamily play critical roles in signaling pathways that control cell growth, differentiation and survival. Recently, several investigators have identified a number of aPKC-interacting proteins whose characterization is helping to unravel the mechanisms of action and functions of these kinases. These interactors include p62, Par-6, MEK5 and Par-4. The details of how these adapters serve to link the aPKCs to different receptor signaling pathways and substrates in response to specific stimuli are crucial not only for developing an understanding of the roles and functions of the aPKCs themselves, but also for more generally establishing a view of how specificity in signal transduction is achieved.
Figures

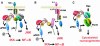

References
-
- Berra E., Diaz-Meco, M.T., Dominguez, I., Municio, M.M., Sanz, L., Lozano, J., Chapkin, R.S. and Moscat, J. (1993) Protein kinase Cζ isoform is critical for mitogenic signal transduction. Cell, 74, 555–563. - PubMed
-
- Bjorkoy G., Perander, M., Overvatn, A. and Johansen, T. (1997) Reversion of Ras- and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase Cλ is accompanied by the loss of constitutive nuclear mitogen-activated protein kinase/extracellular signal-regulated kinase activity. J. Biol. Chem., 272, 11557–11565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

