One RNA polymerase serving two genomes
- PMID: 11258484
- PMCID: PMC1083759
- DOI: 10.1093/embo-reports/kvd086
One RNA polymerase serving two genomes
Abstract
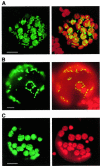
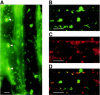
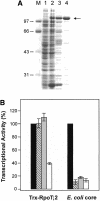
The land plant Arabidopsis thaliana contains three closely related nuclear genes encoding phage-type RNA polymerases (RpoT;1, RpoT;2 and RpoT;3). The gene products of RpoT;1 and RpoT;3 have previously been shown to be imported into mitochondria and chloroplasts, respectively. Here we show that the transit peptide of RpoT;2 possesses dual targeting properties. Transient expression assays in tobacco protoplasts as well as stable transformation of Arabidopsis plants demonstrate efficient targeting of fusion peptides consisting of the N-terminus of RpoT;2 joined to green fluorescent protein to both organelles. Thus, RpoT;2 might be the first RNA polymerase shown to transcribe genes in two different genomes. RNA polymerase activity of recombinant RpoT;2 is uneffected by the inhibitor tagetin, qualifying the gene product of RpoT;2 as a phage-type polymerase.
Figures

References
-
- Akashi K., Grandjean, O. and Small, I. (1998) Potential dual targeting of an Arabidopsis archaebacterial-like histidyl-tRNA synthetase to mitochondria and chloroplasts. FEBS Lett., 431, 39–44. - PubMed
-
- Bechtold N., Ellis, J. and Pelletier, G. (1993). In planta Agrobacterium gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris, 316, 1194–1199.
-
- Börner T., Hedtke, B., Hess, W.R., Legen, J., Herrmann, R.G. and Weihe, A. (1999) Phage-type RNA polymerases in higher plants. In Argyroudi-Akoyunoglou, J.H. and Senger, H. (eds) The Chloroplast: From Molecular Biology to Biotechnology, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 73–78.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

