MCM3AP, a novel acetyltransferase that acetylates replication protein MCM3
- PMID: 11258703
- PMCID: PMC1083822
- DOI: 10.1093/embo-reports/kve026
MCM3AP, a novel acetyltransferase that acetylates replication protein MCM3
Abstract
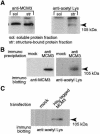
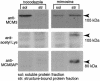
The MCM proteins are essential for the initiation of DNA replication. We have isolated an MCM3-associated protein (MCM3AP) in a two-hybrid screen using MCM3. Here we demonstrate that MCM3AP is an acetyltransferase which acetylates MCM3 and that chromatin-bound MCM3 is acetylated in vivo. The MCM3 acetylase, MCM3AP, is also chromatin-bound. This study also indicates that MCM3AP contains putative acetyl CoA binding motifs conserved within the GCN5-related N-acetyltransferase superfamily. Mutation of those motifs significantly inhibits the MCM3 acetylase activity. Over-expression of MCM3AP inhibits DNA replication, whereas mutation of the acetylase motifs abolishes this effect, suggesting that acetylation plays a role in DNA replication. Taken together, we suggest that MCM3 acetylation is a novel pathway which might regulate DNA replication.
Figures


References
-
- Chen H., Lin, R.J., Xie, W., Wilpitz, D. and Evans, R.M. (1999) Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell, 98, 675–686. - PubMed
-
- Cheung W.L., Briggs, S.D. and Allis, C.D. (2000) Acetylation and chromosomal functions. Curr. Opin. Cell. Biol., 12, 326–333. - PubMed
-
- Coverley D., Wilkinson, H.R., Madine, M.A., Mills, A.D. and Laskey, R.A. (1998) Protein kinase inhibition in G2 causes mammalian Mcm proteins to reassociate with chromatin and restores ability to replicate. Exp. Cell Res., 238, 63–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

